Abstract
Purpose
Many methods exist in the literature for identifying PEEP to set in ARDS patients following a lung recruitment maneuver (RM). We compared ten published parameters for setting PEEP following a RM.
Methods
Lung injury was induced by bilateral lung lavage in 14 female Dorset sheep, yielding a PaO2 100–150 mmHg at FIO2 1.0 and PEEP 5 cmH2O. A quasi-static P–V curve was then performed using the supersyringe method; PEEP was set to 20 cmH2O and a RM performed with pressure control ventilation (inspiratory pressure set to 40–50 cmH2O), until PaO2 + PaCO2 > 400 mmHg. Following the RM, a decremental PEEP trial was performed. The PEEP was decreased in 1 cmH2O steps every 5 min until 15 cmH2O was reached. Parameters measured during the decremental PEEP trial were compared with parameters obtained from the P–V curve.
Results
For setting PEEP, maximum dynamic tidal respiratory compliance, maximum PaO2, maximum PaO2 + PaCO2, and minimum shunt calculated during the decremental PEEP trial, and the lower Pflex and point of maximal compliance increase on the inflation limb of the P–V curve (Pmci,i) were statistically indistinguishable. The PEEP value obtained using the deflation upper Pflex and the point of maximal compliance decrease on the deflation limb were significantly higher, and the true inflection point on the inflation limb and minimum PaCO2 were significantly lower than the other variables.
Conclusion
In this animal model of ARDS, dynamic tidal respiratory compliance, maximum PaO2, maximum PaO2 + PaCO2, minimum shunt, inflation lower Pflex and Pmci,i yield similar values for PEEP following a recruitment maneuver.
Similar content being viewed by others
Introduction
Controversy over the approach to setting PEEP has existed ever since Asbaugh et al. [1] first described the use of PEEP to manage ARDS. Some data suggest that heterogeneous inflation of the lung parenchyma can promote substantial shear stress and strain, leading many investigators to seek methods to make end-expiratory alveolar inflation more homogeneous [2]. Many have considered lung recruitment maneuvers during the initial days of ventilatory support for ARDS [3–12] followed by high PEEP as one method to achieve lung inflation homogeneity. However, debate regarding the value of recruitment maneuvers and the setting of PEEP post lung recruitment persists [13].
Proponents of homogeneous alveolar inflation (“open lung strategy”) point to a substantial number of animal studies showing the benefits of recruitment and PEEP in preventing lung injury [14–17] and human studies showing improvement in gas exchange and lung mechanics [12, 18, 19]. These authors also point out that the ARDS Network strategy of using a PEEP-FIO2 table to set PEEP generally results in relatively low levels of PEEP potentially promoting derecruitment and therefore repetitive end-expiratory alveolar collapse (“atelectrauma”) [20]. Whether higher PEEP may further improve mortality in ARDS is still a matter of debate and will require more randomized trials. To interpret results of clinical trials using different methods of setting “open-lung PEEP”, a comparison of different methods is needed. To date, multiple methods have been proposed [21–24]. Some methods employ a decremental PEEP trial [25], other methods utilize the quasi-static P–V curve to obtain the inflation limb lower Pflex or deflation limb upper Pflex [22, 26, 27] or curve-fitting to calculate the points of maximal compliance increase on the inflation limb (Pmci,i), maximal compliance decrease on the deflation limb (Pmcd,d), or true inflection point of the deflation limb (Fig. 1) [28, 29]. Using CT scanning in a saline lavage model of lung injury in pigs, Suarez-Sipmann et al. [30] demonstrated that measuring dynamic tidal respiratory compliance during a decremental PEEP trial following a recruitment maneuver could accurately identify the PEEP level below which significant derecruitment occurred. Also using CT scanning, Borges et al. [12] showed in humans that using a recruitment maneuver followed by a decremental PEEP trial, the lung could be opened and maintained open.
Examples of different points on the P–V curve that have been proposed for setting PEEP. The lower inflection point of the inflation limb (lower Pflex,i) is identified by the intersection of two lines, one drawn through a region of low compliance at the beginning of inflation and one through the region of highest compliance. A similar method is done for the upper inflection point of the deflation limb (upper Pflex,d). The point of maximum compliance increase on the inflation limb (Pmci,i), point of maximum compliance decrease on the deflation limb (Pmcd,d) and true inflection point of the deflation limb (Pinf,d) are calculated from the curve-fitting parameters of the equation V = a + b/(1 + e −(P − c)/d) [28, 29]
The goal of this study was to determine the relationship among the following parameters in identifying open-lung PEEP measured during a decremental PEEP trial after a RM: the maximum dynamic tidal respiratory compliance, the maximum PO2 + PCO2, the maximum PaO2, the minimum PaCO2, and the minimum shunt. We also compared parameters obtained from a quasi-static pressure–volume (P–V) curve done prior to the recruitment maneuver: inflation lower Pflex, deflation upper Pflex, Pmci,i, Pmcd,d, and Pinf,d. Our hypothesis was that the parameters derived from a decremental PEEP trial should yield similar open-lung PEEP values, whereas values derived from the P–V curve would be different. We tested this hypothesis in a lavage lung injured sheep model. Since the “optimal” or “best” PEEP in ARDS is an intensely debated topic, we chose one method, the maximum dynamic compliance during a decremental PEEP titration as the reference, and compared other methods to this. Our goal was not to propose the best PEEP or the best method for identifying best PEEP in ARDS, but rather to compare the different methods to see if they give similar values.
Methods
Animals and anesthesia
A total of 14 female Dorset sheep (25–35 kg) were studied in the supine position. Sheep were fasted for 24 h then orotracheally intubated during deep halothane mask anesthesia. The external jugular vein was cannulated. After line placement, the anesthetic was switched to total intravenous anesthesia with a loading dose of 10 mg/kg Pentobarbital, 4 mg/kg Ketamine and 0.1 mg/kg Pancuronium. Continuous infusion of Pentobarbital (4 mg/kg/h), Ketamine (8 mg/kg/h) and Pancuronium (0.1 mg/kg/h) were used. An infusion of Lactated Ringer’s solution (20 ml/kg/h) was administered to maintain intravascular volume and was increased as necessary for hypotension.
Monitoring
An 18-gauge catheter was placed into the carotid artery for measurement of arterial blood pressure (ABP) and arterial blood gases (ABG). Arterial and mixed venous blood samples were drawn for blood gas analyses (model 282, Ciba Corning Diagnostics Corp., Norwood, MA, USA). Flow (\( \dot{V} \)) was measured by a heated pneumotachometer (Hans Rudolph, inc) connected to a differential pressure transducer (Validyne MP-45 ± 2 cmH2O). Volume was determined by digital integration of the flow signal. A differential pressure transducer (Validyne MP-45 ± 100 cmH2O) was used to measure airway opening pressure (Pao). Cardiac index (CI) and Pulmonary artery pressure (PAP) was monitored with a 7.5 F pulmonary artery catheter inserted into an external jugular vein (831 HF 7.5, Edwards Life Sciences LLC, Irvine, CA, USA).
All signals (\( \dot{V} \), Pao, ABP, and PAP) were amplified (Model 8805C, Hewlett Packard, Waltham, MA, USA), and converted to digital signals using an analog–digital converter (DI-220, Dataq Instruments Inc., Akron, OH, USA) and recorded at a sampling rate of 100 Hz using data acquisition software (Powerlab, Chart 5 V5.3, ADInstruments). All infusions were given via a volumetric infusion pump. A core temperature of 39°C was maintained with a heating blanket. A gastric tube was placed to ensure abdominal decompression.
Experimental protocol
After intubation, basic ventilator settings were volume control ventilation (VCV) tidal volume (VT) 10 ml/kg, inspiratory to expiratory time (I:E) ratio 1:2, FIO2 1.0 and PEEP 5 cmH2O. Respiratory rate was adjusted to achieve eucapnia (PaCO2 35–45 mmHg). Severe lung injury was then produced by bilateral lung lavage with 1-l instillation of isotonic saline warmed to 39oC, repeated every 30 min until the PaO2 was 100–150 mmHg at FIO2 1.0 and PEEP 5 cmH2O. Care was taken not to exceed a height of 30 cmH2O during saline instillation. Ventilatory rate was adjusted to keep the CO2 35–45 mmHg. Following establishment of lung injury, VCV was continued for 60 min in order to confirm the stability of lung injury. Stable lung injury was defined as a PaO2 change of less than 10% after 60 min.
Baseline settings and P–V curve
After establishment of stable lung injury, ventilation was changed to lung injury baseline ventilation as follows: pressure control ventilation (PCV), PEEP 5 cmH2O, peak pressure 30 cmH2O, inspiratory time 0.6 s, rate to maintain PCO2 35–45 mmHg (about 30–40/min), and FIO2 1.0. Animals were then allowed to stabilize on these settings for 15 minutes followed by data gathering. Following stabilization, a quasi-static inflation and deflation pressure–volume curve to a peak pressure of 50 cmH2O was performed using a 2-l super syringe. Volume measured during the P–V curve was corrected for pressure, temperature, humidity, time, O2 consumption, and CO2 production as previously described [29].
Recruitment maneuvers
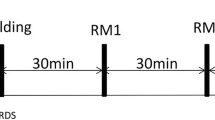
Following the performance of the P–V curve animals were stabilized for 15 min on the following settings (Pre RM) and data gathered: PCV peak pressure 35 cmH2O, PEEP 20 cmH2O, inspiratory time 0.6 s, rate 40/min, FIO2 1.0. The lung was then recruited in three steps alternated with stabilization periods (Fig. 2). After each step data were obtained:
Protocol for study: Baseline, Lung Injury, Pressure–volume (P–V) curve, and the Recruitment Strategy performed under pressure controlled ventilation with PEEP 20 cmH2O, Peak Pressure of 40, 45, and 50 cmH2O (2 min each) alternating with a 5-min stabilization period with a peak pressure of 35 cmH2O. When the PaO2 + PaCO2 > 400 mmHg was achieved, PEEP Titration was performed from 20 to 15 cmH2O (5 min each) in all animals
Step 1: PEEP was set to 20 cmH2O and pressure control set to a peak airway pressure of 40 cmH2O. These settings were maintained for 2 min, followed by a 5-min stabilization period with peak pressure 35 cmH2O. Data were gathered at 2 min if PaO2 + PaCO2 > 400 mmHg.
Step 2: If PaO2 + PaCO2 after step 1 <400 mmHg the PEEP setting remained unchanged and pressure control was increased to obtain a peak airway pressures of 45 cmH2O. This pattern was sustained for 2 min, followed by a 5-min stabilization period with peak pressure 35 cmH2O. Data were gathered at 2 min, if PaO2 + PaCO2 > 400 mmHg.
Step 3: If PaO2 + PaCO2 after steps 1 and 2 <400 mmHg, the PEEP setting remained unchanged and pressure control was increased to obtain a peak airway pressure of 50 cmH2O. These settings were maintained for 2 min, followed by a 5-min stabilization period with peak pressure 35 cmH2O. Data were gathered at 2 min.
If our blood-gas target was not met despite the application of peak inspiratory pressure of 50 cmH2O, the maneuver was terminated and the recruitment was considered incomplete.
PEEP titration
Immediately after the PaO2 + PaCO2 > 400 mmHg, all animals underwent a decremental PEEP-titration in volume control mode. PEEP was decreased in 1 cmH2O steps and maintained at that level for 5 min, before again being reduced by 1 cmH2O. Dynamic tidal respiratory compliance was measured at each step using a V T of 4 ml/kg and a frequency of 40/min. This process was continued until the maximum compliance was achieved as determined by a rise in compliance and then a fall with each PEEP step. This process was similar to previously published trials [12, 30], and we stopped after the maximum dynamic compliance was measured since others have shown that compliance continues to fall once the maximum is achieved [30]. After each step physiologic data were gathered.
Analysis of the P–V curve
Inflation and deflation P–V data were fitted with the equation
This equation, which has been described previously [28], has four fitting parameters: a, in units of volume, representing the lower asymptote; b, in units of volume, representing the distance from a to the upper asymptote, or inspiratory capacity; c, in units of pressure, representing the true inflection point (where concavity changes direction); and d, in units of pressure, representing the distance from c to the zone of high compliance. Using the program DeltaGraph (Red Rock Software, Inc., Salt Lake City, UT, USA), the equation was fitted to the PV data using the Levenberg–Marquardt iterative algorithm to minimize the sum of squared residuals. The algorithm was set to run until the resulting sum of squared residuals changed by <0.0001, yielding estimates of the parameters a, b, c, and d and the best-fit coefficient R 2. Initial guess coefficients were a = 0 L, b = 3 L, c = 20 cmH2O, d = 10 cmH2O. Points of maximum compliance increase or decrease or true inflection points (Fig. 1) falling outside the range of data collected (<0 cmH2O or> the highest data point collected in cm H2O) were not included in the analysis.
In addition, from the raw P–V curve data without curve-fitting, the following parameters were derived: inflation Pflex and deflation Pflex. The lower inflation Pflex was the pressure corresponding to the intersection of two lines (Fig. 1), manually drawn on the inflation curve, corresponding to an upward shift in the first and the second slope of the curve [22, 31]. The upper deflation Pflex (Upper Pflex,d) was obtained using the same methodology. The Pflex parameters were determined by one of authors (MPC).
Variables compared
Using ten different methods, PEEP levels were identified that were candidates for the best “open-lung PEEP”, i.e., the PEEP level that maintained the benefit of a prior lung recruitment. Five methods utilized the production of an optimal physiological response and five were points derived from the pressure–volume curve.
-
1.
Best dynamic tidal respiratory compliance—maximum value;
-
2.
best PaO2 + PaCO2—maximum value;
-
3.
best PaO2—maximum value;
-
4.
best PaCO2—minimum value;
-
5.
best shunt—minimum value
-
6.
Inflation lower Pflex (Lower Pflex,i)
-
7.
Deflation upper Pflex (Upper Pflex,d)
-
8.
Point of maximal compliance decrease on deflation limb (Pmcd,d = c + 1.317d)
-
9.
True inflection point deflation limb (where concavity changes direction, Pinf,d = c)
-
10.
Point of maximal compliance increase on inflation limb (Pmci.i = c − 1.317d)
Since there is no standard definition of “open-lung” PEEP, we used best dynamic tidal respiratory compliance as the reference value, and physiologic parameters measured during the decremental PEEP trial were recorded at all PEEP levels but only reported for the PEEP at the maximum dynamic tidal respiratory compliance.
Statistical analysis
All data are presented as mean ± SD. Repeated-measures ANOVA was used for comparison of all variables collected multiple times during the protocol and then contrast deviation was applied comparing the means of all variables. Significance was defined as P < 0.05 (two-tailed).
Results
Characteristics of the animals
Seventeen female Dorset sheep (25–30 kg) fasted for 24 h were studied. Three animals were excluded because they did not meet our pre-defined blood-gas target (PaO2 + PaCO2 > 400 mmHg). It was necessary to apply 50 cmH2O peak pressure in ten animals to achieve full recruitment; 45 cmH2O in two animals and 40 cmH2O in two animals. No animal was fully recruited with 35 cmH2O peak pressure (Fig. 2).
Decremental PEEP titration
For the determination of open-lung PEEP, dynamic tidal respiratory compliance, PaO2, PaO2 + PaCO2 and shunt, and inflation Pflex and Pmci,i were statistically indistinguishable (Fig. 3). The open-lung PEEP values obtained using the deflation upper Pflex and Pmcd,d were significantly higher and the Pinf,d and PaCO2 were significantly lower than the other variables (Fig. 3).
Open-lung PEEP identified by ten different methods: Compliance, PaO2 + PaCO2, PaO2, PaCO2, shunt, inflation lower Pflex (Lower Pflex,i), deflation upper Pflex (Upper Pflex,d), point of maximum compliance increase on inflation (Pmci,i), point of maximum compliance decrease on deflation (Pmcd,d), and true inflection point of the deflation limb (Pinf,d). The bars represents mean values, the open circles are actual values for each animal. *P < 0.05 compared to all other variables
Physiologic variables
Table 1 shows hemodynamic, plateau pressure (Pplat) and blood-gas measurements obtained during the protocol at four time points: baseline, injury, recruitment, and during the decremental PEEP trial. Variables used for comparison during the decremental PEEP trial were the parameters recorded at the maximum dynamic tidal respiratory compliance. There were no statistically significant differences in any of the monitored values compared to baseline during the protocol except for PaO2, PaCO2 and shunt fraction.
Discussion
The primary finding of this study is that dynamic tidal respiratory compliance, maximum PaO2, maximum PaO2 + PaCO2, minimum shunt, inflation lower Pflex and Pmci,i yielded similar values for open-lung PEEP.
In swine Suarez-Sipman et al. [30] identified dynamic tidal respiratory compliance as the best method of identifying optimal PEEP post lung recruitment. They determined that dynamic tidal respiratory compliance, PaO2, and shunt fraction could be used to identify the PEEP level that was associated with an open lung as defined by CT scan. They emphasized that compliance was the easiest maneuver to use at the bedside because it rapidly equilibrates following PEEP titration and could be easily assessed on all mechanical ventilators. They also showed that maximal lung recruitment and the selection of optimal decremental PEEP almost entirely eliminated tidal lung recruitment minimizing the possibility of atelectrauma when this procedure was followed. Our study adds to these data in that we found other measurements (PaO2 + PaCO2, inflation lower Pflex and Pmci,i) that performed similarly to dynamic tidal respiratory compliance, PaO2 and shunt fraction.
Borges et al. used PaO2 to determine open-lung PEEP following their maximal recruitment maneuver [12]. In all 26 patients studies determining PEEP using this methodology sustained the benefit of the recruitment maneuver throughout their post-recruitment observation period of up to 6 h. Similarly, Tugrul et al. [19] used PaO2 as the determinant of optimal PEEP following lung recruitment during a decremental PEEP trial and also sustained the benefits for their full observation period (6 h). Girgis et al. [18] who observed the same results, however, used SpO2 during their decremental PEEP trial. Our data demonstrate that irrespective of whether one uses maximum PaO2, maximum PaO2 + PaCO2, minimum shunt, maximum dynamic tidal respiratory compliance, inflation lower Pflex, or Pmci,i, the resulting open-lung PEEP will be nearly identical.
Of the decremental PEEP trial variables, only the use of minimum PaCO2 to determine open-lung PEEP was significantly different, and lower, than the other variables. This difference may reflect overdistension at PEEP levels that improve oxygenation. Inasmuch as current goals in ARDS are to balance overdistension and alveolar collapse and re-opening, the fact that open-lung PEEP is higher using oxygenation parameters, might lead one to set PEEP that would limit alveolar collapse, but at the expense of overdistension. However, global overdistension can be avoided by limiting driving pressures to 10–12 cmH2O and end inspiratory plateau pressures to <30 cmH2O, while accepting permissive hypercapnia. Other investigators have suggested other parameters not tested in this study, such as the stress index, that may better balance the goals of optimizing the balance between overdistension and alveolar collapse and re-opening [32].
Pmci,i has been compared to the inspiratory lower Pflex in human ARDS [29]. Harris et al. [29] found Pflex was a poor estimate of Pmci,i for individual values, although the mean difference between the two was only 0.61 cmH2O. Our mean difference between these variables was small (0.08) and the individual relationship between these values was better than that reported by Harris. The reason for this may be twofold: first, ours is a lung injury model in sheep and the determination of Pflex was performed by only one person rather than seven. Thus, both biological and methodological variance should have been minimized in the present study. In contrast to the study by Harris et al., we found that Pflex + 2 cmH2O did not predict the true inflection point of the deflation limb (Pinf,d). This may also be a result of differences in the saline lavage lung injury model when compared to human ARDS. Regardless of the reason, it is interesting that an inflation parameter on the P–V curve seems to be equally predictive of open-lung PEEP determined from a deflation decremental PEEP trial.
There are limitations to this study that must be acknowledged. It was performed using a saline lavage, sheep model of ARDS, which is a highly recruitable lung injury model. Therefore, the results here cannot necessarily be generalized to human ARDS. Also, the results may not be the same for other models of lung injury where the lung injury is characterized by substantial alveolar flooding [32]. We did not randomize the order of the PV curve and the decremental PEEP trial which could have introduced a systematic bias. In addition, the decremental PEEP trial was begun at 20 cmH2O; as a result, we cannot be certain that the open-lung PEEP was not higher than this in some animals potentially increasing the variability of the parameters obtained during the decremental trial. This was done to insure hemodynamic stability throughout the study because we did experience marked instability at higher PEEP levels in pilot studies. In addition, our average open-lung PEEP (19 cmH2O) was higher than that obtained by Suarez-Sipmann [30], using a swine lavage injured model where decremental PEEP titration began at 24 cmH2O (14 cmH2O) and was equivalent to that observed by Borges [12] in patients with ARDS where PEEP titration began at 25 cmH2O and open-lung PEEP was 18 cmH2O. The fact that the PV curve was not limited to 20 cmH2O means that if one could ignore hypotension and acidemia during the decremental PEEP trial and start at higher PEEP levels, perhaps the comparisons of open-lung PEEP with the PV-derived parameters could have been different. However, since hemodynamic considerations must be taken into account clinically, this comparison would have questionable significance. Also, one could argue that the narrow range of PEEP studied biases the results towards the null hypothesis, i.e., finding no difference between the methods, but in all animals, the maximum dynamic tidal respiratory compliance was found at PEEP levels above 15 cmH2O, and it has been shown to decrease progressively once a peak is found [30].
In conclusion, this study shows that in a surfactant deficient lung lavage model of ARDS, open-lung PEEP can be consistently identified using a decremental PEEP trial after a recruitment maneuver by measuring maximum dynamic tidal respiratory compliance, maximum PaO2, maximum PaO2 + PaCO2, or minimum shunt, or by performing a quasi-static P–V curve and measuring inflation Pflex and Pmci,i.
References
Ashbaugh DG, Bigelow DB, Petty TL, Levine BE (1967) Acute respiratory distress in adults. Lancet 2:319–323
Mead J, Takishima T, Leith D (1970) Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol 28:596–608
Slutsky AS (1993) Barotrauma and alveolar recruitment. Intensive Care Med 19:369–371
Marini JJ, Amato MBP (1997) Lung recruitment during ARDS. In: Marini JJ, Evans TW (eds) Acute lung injury. Springer, Berlin, pp 236–257
Medoff BD, Harris RS, Kesselman H, Venegas J, Amato MB, Hess D (2000) Use of recruitment maneuvers and high-positive end-expiratory pressure in a patient with acute respiratory distress syndrome. Crit Care Med 28:1210–1216
Marini JJ, Amato MB (2000) Lung recruitment during ARDS. Minerva Anestesiol 66:314–319
Richards GA, Hopley M, Kacmarek RM, Fujino Y, Amato M (2002) Successful recruitment. Crit Care Med 30:2169
Barbas CS, de Matos GF, Okamoto V, Borges JB, Amato MB, de Carvalho CR (2003) Lung recruitment maneuvers in acute respiratory distress syndrome. Respir Care Clin N Am 9:401–418
Barbas CS, de Matos GF, Pincelli MP, da Rosa Borges E, Antunes T, de Barros JM, Okamoto V, Borges JB, Amato MB, de Carvalho CR (2005) Mechanical ventilation in acute respiratory failure: recruitment and high positive end-expiratory pressure are necessary. Curr Opin Crit Care 11:18–28
Ferguson ND, Chiche JD, Kacmarek RM, Hallett DC, Mehta S, Findlay GP, Granton JT, Slutsky AS, Stewart TE (2005) Combining high-frequency oscillatory ventilation and recruitment maneuvers in adults with early acute respiratory distress syndrome: the Treatment with Oscillation and an Open Lung Strategy (TOOLS) Trial pilot study. Crit Care Med 33:479–486
Lapinsky SE, Mehta S (2005) Bench-to-bedside review: recruitment and recruiting maneuvers. Crit Care 9:60–65
Borges JB, Okamoto VN, Matos GF, Caramez MP, Arantes PR, Barros F, Souza CE, Victorino JA, Kacmarek RM, Barbas CS, Carvalho CR, Amato MB (2006) Reversibility of lung collapse and hypoxemia in early acute respiratory distress syndrome. Am J Respir Crit Care Med 174:268–278
Brower RG, Morris A, MacIntyre N, Matthay MA, Hayden D, Thompson T, Clemmer T, Lanken PN, Schoenfeld D (2003) Effects of recruitment maneuvers in patients with acute lung injury and acute respiratory distress syndrome ventilated with high positive end-expiratory pressure. Crit Care Med 31:2592–2597
Muscedere JG, Mullen JBM, Slutsky AS (1994) Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med 149:1327–1334
Dreyfuss D, Saumon G (1998) Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 157:294–323
McCulloch PR, Forkert PG, Froese AB (1988) Lung volume maintenance prevents lung injury during high frequency oscillatory ventilation in surfactant-deficient rabbits. Am Rev Respir Dis 137:1185–1192
Bond DM, Froese AB (1993) Volume recruitment maneuvers are less deleterious than persistent low lung volumes in the atelectasis-prone rabbit lung during high-frequency oscillation. Crit Care Med 21:402–412
Girgis K, Hamed H, Khater Y, Kacmarek RM (2006) A decremental PEEP trial identifies the PEEP level that maintains oxygenation after lung recruitment. Respir Care 51:1132–1139
Tugrul S, Akinci O, Ozcan PE, Ince S, Esen F, Telci L, Akpir K, Cakar N (2003) Effects of sustained inflation and postinflation positive end-expiratory pressure in acute respiratory distress syndrome: focusing on pulmonary and extrapulmonary forms. Crit Care Med 31:738–744
Acute Respiratory Distress Syndrome Network (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 342:1301–1308
Lachmann B (1992) Open up the lung and keep the lung open. Intensive Care Med 18:319–321
Amato MB, Barbas CS, Medeiros DM, GdP Schettino, Lorenzi Filho G, Kairalla RA, Deheinzelin D, Morais C, EdO Fernandes, Takagaki TY et al (1995) Beneficial effects of the “open lung approach” with low distending pressures in acute respiratory distress syndrome. A prospective randomized study on mechanical ventilation. Am J Respir Crit Care Med 152:1835–1846
Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR (1998) Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 338:347–354
Marini JJ (2003) How to recruit the injured lung. Minerva Anestesiol 69:193–200
Hickling KG (2001) Best compliance during a decremental, but not incremental, positive end-expiratory pressure trial is related to open-lung positive end-expiratory pressure. A mathematical model of acute respiratory distress syndrome lungs. Am J Respir Crit Care Med 163:69–78
Gattinoni L, D’Andrea L, Pelosi P, Vitale G, Pesenti A, Fumagalli R (1993) Regional effects and mechanism of positive end-expiratory pressure in early adult respiratory distress syndrome. JAMA 269:2122–2127
Ranieri VM, Giuliani R, Fiore T, Dambrosio M, Milic-Emili J (1994) Volume-pressure curve of the respiratory system predicts effects of PEEP in ARDS: “occlusion” versus “constant-flow” technique. Am J Respir Crit Care Med 149:19–27
Venegas JG, Harris RS, Simon BA (1998) A comprehensive equation for the pulmonary pressure–volume curve. J Appl Physiol 84:389–395
Harris RS, Hess DR, Venegas JG (2000) An objective analysis of the pressure–volume curve in the acute respiratory distress syndrome. Am J Respir Crit Care Med 161:432–439
Suarez-Sipmann F, Bohm SH, Tusman G, Pesch T, Thamm O, Reissmann H, Reske A, Magnusson A, Hedenstierna G (2007) Use of dynamic compliance for open lung positive end-expiratory pressure titration in an experimental study. Crit Care Med 35:214–221
Gattinoni L, Pesenti A, Avalli L, Rossi F, Bombino M (1987) Pressure-volume curve of total respiratory system in acute respiratory failure. Computed tomographic scan study. Am Rev Respir Dis 136:730–736
Ranieri VM, Grasso S, Fiore T, Giuliani R (1996) Auto-positive end-expiratory pressure and dynamic hyperinflation. Clin Chest Med 17:379–394
Acknowledgments
This study was funded by departmental funds. Robert Kacmarek has received research grants and honorarium for lecturing from Puritan Bennett, Maquet Medical, Hamilton Medical, and Respironics, Inc. and is a consultant for SpaceLabs Healthcare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caramez, M.P., Kacmarek, R.M., Helmy, M. et al. A comparison of methods to identify open-lung PEEP. Intensive Care Med 35, 740–747 (2009). https://doi.org/10.1007/s00134-009-1412-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-009-1412-9