Abstract
Purpose
Transfusion-related acute lung injury (TRALI) occurs more often in critically ill patients than in a general hospital population, possibly due to the presence of underlying inflammatory conditions that may prime pulmonary neutrophils. Mechanical ventilation may be a risk factor for developing TRALI. We examined the influence of mechanical ventilation (MV) on the development of TRALI, combining a murine MV model causing ventilator-induced lung injury with a model of antibody-induced TRALl.
Methods
BALB/c mice (n = 84) were ventilated for 5 h with low (7.5 ml/kg) or high (15 ml/kg) tidal volume, a positive end-expiratory pressure of 2 cm H2O and a fraction of inspired oxygen of 50%. After 3 h of MV, TRALI was induced by infusion of MHC-I antibodies (4.5 mg/kg); controls received vehicle. Non-ventilated animals receiving vehicle, isotype or MHC-I antibodies served as additional controls.
Results
All animals receiving MHC-I antibodies developed TRALI within 2 h. In mice in which TRALI was induced, MV with low tidal volumes aggravated pulmonary injury, as evidenced by an increase in neutrophil influx, pulmonary and systemic levels of cytokines and lung histopathological changes compared to unventilated controls. The use of high tidal volume ventilation resulted in a further increase in protein leakage and pulmonary edema.
Conclusions
Mechanical ventilation (MV) synergistically augmented lung injury during TRALI, which was even further enhanced by the use of injurious ventilator settings. Results suggest that MV may be a risk factor for the onset of TRALI and may aggravate the course of disease.
Similar content being viewed by others
Introduction
Transfusion-related acute lung injury (TRALI) is the leading cause of transfusion-related morbidity and mortality [10, 24]. Traditionally, TRALI is viewed as a rare complication of blood transfusion. However, this may not hold true for critically ill patients. An accumulating number of studies report a high incidence of TRALI in the critically ill patient population [3, 8, 20, 27, 29, 31–33], with incidences ranging from 5.1–8% per transfused patient, compared to 0.02–0.16% per transfused patient in the general hospital population [8, 20, 27, 31–33]. These differences may be explained by the proposed “two hit” mechanism of TRALI [21]. The “first hit” is the underlying condition of the patient which results in priming of the pulmonary neutrophils (e.g. sepsis, pneumonia). The “second hit” is caused by the transfusion of antibodies directed against the recipient’s antigens or biologically active lipids which accumulate during storage. As critically ill patients frequently suffer from an underlying condition, this patient group may be more susceptible to developing TRALI.
Although mechanical ventilation (MV) may be an inevitable procedure, MV can induce or aggravate lung injury, referred to as ventilator induced lung injury (VILI). Low tidal volume ventilation reduces mortality in patients with acute lung injury [1], indicating that ventilator settings influence pulmonary injury. Notably, ventilation with low tidal volumes also induces neutrophil-mediated lung injury [4, 34, 36]. Therefore, it can be hypothesized that MV may prime pulmonary neutrophils, rendering the lungs more susceptible to a TRALI reaction. In an observational study, as many as 33% of mechanically ventilated critically ill patients developed lung injury within 48 h after transfusion [7]. Also, transfusion and large tidal volumes appear to have a synergistic effect on the development of ALI [6]. However, no direct evidence exists as to whether MV may be a priming factor for the onset of TRALI, or whether it may aggravate the course of a TRALI reaction.
Although it is usually stated that the prognosis of TRALI is good [19], recent studies show that TRALI is associated with increased morbidity and mortality in the critically ill [3, 8, 20, 27]. Therefore, it is important to elucidate the pathogenesis of TRALI in this patient group. To investigate whether MV affects the development and course of a TRALI reaction, we adopted a well-established model of VILI, in which TRALI was induced by infusion of MHC-I antibodies [9, 17, 36].
Methods
The study was approved by the Animal Care and Use Committee of the Academic Medical Center at the University of Amsterdam, Amsterdam, The Netherlands. Animal procedures were carried out in compliance with Institutional Standards for Human Care and Use of Laboratory Animals. In the online-supplement, the “Method” section is described in more detail.
MHC-I mAb
A hybridoma (34-1-2S) was purchased from the American Type Culture Collection that produces mAb against H2Kd (IgG2a, κ), which have previously been shown to induce TRALI in an animal model [17]. As isotype matched antibodies we used an IgG2a, κ producing hybridoma (CRL-1908), from the American Type Culture Collection.
Mice
Experiments were performed with healthy male BALB/c mice (n = 84) (Charles River, Someren, the Netherlands), aged 8–10 weeks and weights 19–25 g, randomly assigned to 7 groups (n = 12 per group) (Fig. 1, online-supplement). Three groups (infusion of PBS, infusion of isotype antibody, infusion of MHC-I class antibody) served as non-ventilated controls and were killed after 2 h. The other animals were mechanically ventilated with two different strategies for 5 h and received either PBS infusion or MHC-I class infusion after 3 h of ventilation.
Mechanical ventilation strategies and monitoring
Animals were anesthetized as previously described [35, 36]. Mice were placed in a supine position, connected to a ventilator (Servo 900 C, Siemens, Sweden) and pressure-controlled ventilated with either an inspiratory pressure of 10 cm H2O (resulting in lung-protective V T ~ 7.5 mL/kg; low tidal) or an inspiratory pressure of 18 cm H2O (resulting in injurious V T ~ 15 mL/kg; high tidal). Respiratory rate was set at 110 breaths/min and 70 breaths/min with low tidal and high tidal, respectively. After 3 h, the jugular vein was isolated. Using a 30-gauge sterile needle, venous blood was aspirated from the jugular vein to verify intravascular placement of the needle and to remove a sample of blood (~200 μl). Mice were given an i.v. volume-matched injection (150–250 μl) of either MHC I mAb (4.5 mg/kg) or PBS. Systolic blood pressure and heart rate were non-invasively monitored using a murine tail-cuff system (AD Instruments, Spenbach, Germany) and recorded on a data acquisition system (PowerLab/4SP, ADInstruments).
Study groups and sampling
Non-ventilated control mice were spontaneously breathing and were killed after 2 h. LV T-mice and HV T-mice were mechanically ventilated for 5 h and then killed. Subsequently, in 6 animals, bronchoalveolar lavage fluid (BALF) was obtained from the right lung and cell counts were determined using a hemacytometer (Beckman Coulter, Fullerton, CA). Differential counts were done on cytospin preparations stained with a modified Giemsa stain, Diff-Quick (Dade Behring AG, Düdingen, Switzerland). Supernatant was stored at −80°C for total protein level and cytokine measurement. The left lung was used to determine the wet to dry ratio. Another 6 mice were used for blood gas analysis from blood sampled from the carotid artery and for histopathology of the lungs (fixed in 4% formalin and embedded in paraffin).
Assays
Blood gas analysis was done in a Rapidlab 865 blood gas analyzer (Bayer, Mijdrecht, the Netherlands). The other blood samples were centrifuged and the supernatants were aliquoted and frozen at −20°C. Total protein levels in BALF were determined using a Bradford Protein Assay Kit (OZ Biosciences, Marseille, France) according to the manufacturer’s instructions with bovine serum albumin as standard. Cytokine and chemokine levels were measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions. Tumor necrosis factor (TNF)-α, interleukin (IL)-6, macrophage inflammatory protein (MIP)-2 and keratinocyte-derived chemokine (KC) assays were obtained from R&D Systems (Abingdon, UK).
Statistical analysis
All data in the results are expressed as mean ± sem or median ± interquartile range, where appropriate. To detect differences between groups, paired T test, Dunnett’s method or Mann–Whitney U test was used when appropriate. A p value of <0.05 was considered statistically significant. All statistical analyses were carried out using SPSS 12.0.2 (SPSS, Chicago, IL).
Results
Hemodynamic and ventilatory monitoring
In non-ventilated control animals, respiratory rate did not change after infusion of vehicle or isotype control antibody (Fig. 2, online-supplement). All control animals survived the 2 h. In contrast, mice infused with MHC-I antibodies showed respiratory distress and tachypnea shortly after infusion. Survival after 2 h in non-ventilated animals with TRALI was reduced to 60%.
All ventilated animals survived the 5 h of MV. Blood gas analysis showed that animals achieved adequate gas exchange. Systolic arterial pressure and heart rate remained stable in all animals throughout the experiment.
Infusion of MHC-I antibodies results in TRALI in non-ventilated animals
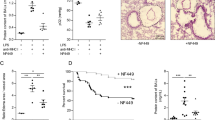
MHC-I antibodies induced TRALI in non-ventilated animals, as indicated by an increase in respiratory rate (Fig. 2, online-supplement, p < 0.01), accompanied by an increase in pulmonary wet-to-dry ratio (Fig. 1, p < 0.01), pulmonary neutrophil influx (Tables 1, 2, p < 0.05) and protein leakage of the lungs (Fig. 2, p < 0.01) compared to vehicle and isotype controls. Histopathological changes showed an increase in pulmonary neutrophil sequestration compared to controls (Table 2; Fig. 3).
Total protein leakage in bronchoalveolar lavage fluid (BALF). Non-ventilated animals receiving PBS, isotype control antibody or MHC-I antibody. Low V T (mice ventilated for 5 h with a tidal of 7.5 ml/kg) and High V T (mice ventilated for 5 h with a tidal of 15 ml/kg) received PBS or MHC-I antibody infusion. * p < 0.05, ** p < 0.01
Transfusion of MHC-I antibodies aggravates ventilator induced lung injury. Histologic sections of hematoxylin and eosin stained mice lungs at 100× magnification. Non-ventilated animals receiving PBS, or MHC-I antibody. Low V T (mice ventilated for 5 h with a tidal of 7.5 ml/kg) and High V T (mice ventilated for 5 h with a tidal of 15 ml/kg) received PBS or MHC-I antibody infusion a PBS non-ventilated; b MHC-I non-ventilated; c PBS and Low V T; d PBS and High V T; e MHC-I and Low V T; f MHC-I and High V T. Normal vasculature (a). Neutrophils sequestrated in the vasculature (arrow) (b–f). Increased pulmonary edema and neutrophil extravasation (d–f)
Pulmonary levels of IL-6 and KC were increased in non-ventilated mice challenged with MHC-I infusion compared to controls (Fig. 4, p < 0.01). The antibodies also induced a systemic inflammatory reaction, increasing plasma levels of IL-6, KC and MIP-2 compared to controls (p < 0.01), whereas TNF did not change. (Fig. 5).
Interleukin (IL)-6 and keratinocyte-derived chemokine (KC) concentrations in the bronchoalveolar lavage fluid (BALF). Non-ventilated animals receiving PBS, isotype control antibody or MHC-I antibody. Low V T (mice ventilated for 5 h with a tidal of 7.5 ml/kg) and High V T (mice ventilated for 5 h with a tidal of 15 ml/kg) received PBS or MHC-I antibody infusion. * p < 0.05 and ** p < 0.01
Interleukin (IL)-6, keratinocyte-derived chemokine (KC), tumor necrosis factor (TNF) and MIP-2 concentrations in the plasma. Non-ventilated animals receiving PBS, isotype control antibody or MHC-I antibody. Low V T (mice ventilated for 5 h with a tidal of 7.5 ml/kg) and High V T (mice ventilated for 5 h with a tidal of 15 ml/kg) received PBS or MHC-I antibody infusion. * p < 0.05 and ** p < 0.01
Mechanical ventilation induces lung injury in previously healthy lungs
Mechanical ventilation (MV) with high tidal volumes, but not with low tidal volumes, resulted in an increased wet-to-dry ratio, accompanied by an increased neutrophil influx and protein leakage in the BALF compared to non-ventilated controls (p < 0.01) (Figs. 1, 2; Table 2) as well as a higher histopathology score (Table 2; Fig. 3, p < 0.05).
Mechanical ventilation (MV) with high tidal volumes, but not with low tidal volumes, resulted in increased pulmonary levels of IL-6 and KC compared to non-ventilated controls (Fig. 4, p < 0.01). MV with high tidal volumes also induced a systemic inflammatory reaction, increasing plasma level of IL-6, KC, MIP-2 and TNF compared to non-ventilated controls (Fig. 5, p < 0.01). MV with low tidal volumes resulted only in increased plasma levels of IL-6 compared to non-ventilated controls.
Mechanical ventilation with protective tidal volumes aggravates lung injury induced by MHC-I antibodies
We investigated the effect of combining MV with protective ventilation settings with the TRALI model. MV with low tidal volumes in animals challenged with MHC-I antibodies resulted in an increased pulmonary neutrophil influx compared to non-ventilated animals challenged with MHC-I antibodies, together with a higher lung injury score (Table 2, p < 0.05; Fig. 3), but did not aggravate pulmonary protein leakage (Fig. 2) nor pulmonary wet-to-dry ratio (Fig. 1). MV with low tidal volume increased pulmonary levels of KC in mice challenged with MHC-I antibody infusion compared to non-ventilated animals challenged with the MHC-I antibody (Fig. 4, p < 0.01), with a non-significant increase in pulmonary levels of IL-6. MV with low tidal volume also aggravated the systemic inflammatory reaction after MHC-I antibody infusion, resulting in increased plasma levels of KC, IL-6 and MIP-2 compared to non-ventilated animals challenged with MHC-I antibody infusion (Fig. 5, p < 0.05).
Mechanical ventilation with injurious tidal volume ventilation further aggravates lung injury induced by MHC-I antibodies
Next, to determine whether injury induced by mechanical ventilation contributes to the course of a TRALI reaction, the effect of injurious ventilation settings was studied in the TRALI model. MV with high tidal volumes induced an increase in wet-to-dry ratio in animals challenged with MHC-I antibodies compared to MV with low tidal volumes and non-ventilated controls challenged with MHC-I antibodies (Fig. 1, p < 0.01). Also, MV with high tidal volumes in animals challenged with MHC-I antibodies increased neutrophil influx (Table 2, p < 0.05), protein leakage in the BALF (Fig. 2, p < 0.01) and histopathology score (Table 2, p < 0.01) compared to non-ventilated animals challenged with MHC-I antibodies. Although not reaching statistical significance, protein leakage and neutrophil influx in the BALF was higher in the animals receiving MV with high tidal volumes and MHC-I antibodies compared to MV with low tidal volumes and MHC-I antibodies.
Mechanical ventilation (MV) with high tidal volumes increased pulmonary levels of IL-6 (Fig. 4, p < 0.05) and KC (Fig. 4, p < 0.001) compared to non-ventilated controls challenged with MHC-I antibodies. MV with high tidal volume also aggravated the systemic inflammatory reaction after MHC-I antibody infusion, increasing plasma levels of IL-6, KC and TNF compared to non-ventilated animals after MHC-I antibody infusion (Fig. 5, p < 0.01). Although not reaching statistical significance, pulmonary levels of IL-6 and KC and systemic levels of IL-6, MIP-2 and TNF were higher in the animals receiving MV with high tidal volumes and MHC-I antibodies compared to MV with low tidal volumes and MHC-I antibodies.
Discussion
We describe a model of antibody-mediated TRALI in a clinically relevant model of mechanical ventilation. MV synergistically augmented lung injury during TRALI, which was even further enhanced by the use of high tidal volumes. These findings support the concept that MV aggravates the pulmonary and systemic course of a TRALI reaction. We postulate that MV may serve as a priming factor, thereby rendering critically ill patients susceptible for a TRALI reaction after receiving a blood transfusion.
In this study, MV synergistically worsened histopathology, pulmonary edema, neutrophil influx and pulmonary and systemic inflammation in MHC-I antibody challenged animals, even with the use of ‘protective’ ventilator settings that did not induce lung injury. In line with this, mechanical stress induced by MV is characterized by a pro-inflammatory response. Such a response may be present even in protective ventilator settings, i.e. with settings that do not cause overt lung injury [26, 36]. Neutrophils recruited to the pulmonary compartment by the ventilator have been found to show evidence of priming [25], resembling a “first hit” in TRALI models. Thereby, during MV, pulmonary neutrophils may be more susceptible to the detrimental effects of the antibodies, resulting in activation after transfusion and the clinical symptoms of TRALI. In line with these findings, we found that MV with low tidal volumes augmented injury inflicted by TRALI antibodies compared to unventilated animals. In particular, neutrophil-influx was enhanced, as were mediators which are released by neutrophils such as pulmonary and systemic inflammation. Mechanical ventilation may predispose patients to TRALI, which may account, at least in part, for the high incidence of TRALI among the critically ill [8, 20, 27].
Mechanical ventilation (MV) with injurious settings causing VILI and antibody-induced TRALI synergistically induced lung injury in this study, suggestive of a “two hit” phenomenon in which MV is the “first hit” and the antibodies are the “second hit”. The “two hit” phenomenon in TRALI has been described after infusion of biological response modifiers that have accumulated during storage of cell-containing blood products, resulting in TRALI in primed lungs. Transfusion of the supernatant of stored blood products caused lung injury after priming with a “first hit” of endotoxin (LPS), but not in the absence of a “first hit” [12, 22, 23]. However, the “two hit” phenomenon has infrequently been described in immune-mediated TRALI [12, 16]. An explanation may be a lack of case reports. The original case description of TRALI involved patients developing acute respiratory failure after transfusion of a plasma product, in whom donor antibodies against leukocyte antigens in the recipient have been linked with the TRALI symptoms. Since then, reports of TRALI cases predominantly describe these “classic” antibody-mediated TRALI symptoms, in which other ALI risk factors are absent [5, 13–15, 18]. In line with our results suggesting that a “two hit” phenomenon may also be present in immune-mediated TRALI, a recent previous experiment showed that antibodies could induce a TRALI reaction in the presence, but not in the absence, of LPS as a priming factor [16].
Our results underscore the relevance of low tidal volume ventilation. Although low tidal volume is now strongly recommended [1], it is still not widely implemented in ALI patients [11]. Our study demonstrates that high tidal ventilation may prime the lungs, thereby lowering the threshold to develop or worsen TRALI. This suggests that the application of low tidal volumes in patients exposed to the risk of a blood transfusion is rational. Another clinical implication pertains to the reporting of TRALI to the Blood Bank. Our results suggest that antibodies in combination with a priming factor may increase lung injury to a clinically significant degree. However, medical disciplines involved in diagnosing and reporting of TRALI consider an inflammatory condition prior to the transfusion a reason to withhold from reporting a suspected TRALI-case [30]. Our results may increase the awareness of critical care specialists, haematologists and transfusion medicine experts that TRALI may also occur in the presence of another ALI risk factor. Results also underline the importance of reporting a suspected TRALI case to the blood bank for analysis of neutrophil and leukocyte antibodies, in order to exclude implicated donors in an effort to prevent future TRALI reactions.
Plasma from multiparous donors is associated with the onset of TRALI, as these donors have been sensitized during labour, resulting in a higher incidence of the presence of leukocyte and/or neutrophil antibodies compared to plasma from male donors. From our results it can be speculated that transfusion with male only plasma may reduce the amount of lung injury in patients with a pro-inflammatory state, such as critically ill patients. In line with this, excluding female donors for high volume plasma components in the UK and The Netherlands reduced the onset of TRALI in several critically ill patient populations [2, 28, 37]. However, deferring women from plasma donation did not completely prevent the onset of TRALI. Therefore, a restrictive transfusion policy remains mandatory. Further studies are needed to identify the effect of male only plasma and restrictive transfusion guidelines of high plasma volume blood products in the critically ill on the occurrence of respiratory complications.
This study has several limitations. First, we used only a component of the blood product, which does not reflect the clinical situation. Second, not all endpoints of lung injury showed a significant difference between the vehicle and MHC-I high tidal ventilation group. This may be due to a type II error, using only 6 animals per group. However, the lung injury endpoints which did not reach statistical significance all showed a clear trend towards a difference.
Conclusion
We developed a clinically relevant model of combining mechanical ventilation and TRALI, in which mechanical ventilation served as a “first hit”, synergistically aggravating lung injury during TRALI, an effect that was augmented by the use of lung injurious mechanical ventilation settings. Our results suggest that in patients at risk for receiving a blood transfusion, even in the absence of lung injury, protective low tidal ventilation is a rational approach.
References
(2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 342:1301–1308
(2005) Shot Annual report 2005. http://www.shotuk.org. SHOT annual report
Benson AB, Moss M, Silliman CC (2009) Transfusion-related acute lung injury (TRALI): a clinical review with emphasis on the critically ill. Br J Haematol 147:431–443
Choi G, Wolthuis EK, Bresser P, Levi M, van der Poll T, Dzoljic M, Vroom MB, Schultz MJ (2006) Mechanical ventilation with lower tidal volumes and positive end-expiratory pressure prevents alveolar coagulation in patients without lung injury. Anesthesiology 105:689–695
Fung YL, Williams BA (2006) TRALI in 2 cases of leukemia. J Pediatr Hematol Oncol 28:391–394
Gajic O, Dara SI, Mendez JL, Adesanya AO, Festic E, Caples SM, Rana R, St SJ, Lymp JF, Afessa B, Hubmayr RD (2004) Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med 32:1817–1824
Gajic O, Rana R, Mendez JL, Rickman OB, Lymp JF, Hubmayr RD, Moore SB (2004) Acute lung injury after blood transfusion in mechanically ventilated patients. Transfusion 44:1468–1474
Gajic O, Rana R, Winters JL, Yilmaz M, Mendez JL, Rickman OB, O’byrne MM, Evenson LK, Malinchoc M, Degoey SR, Afessa B, Hubmayr RD, Moore SB (2007) Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. Am J Respir Crit Care Med 176:886–891
Hicks WA (2004) Investigation of the pathogenesis of transfusion related acute lung injury in a unique murine model. Dissertation, University of Cincinnati
Holness L, Knippen MA, Simmons L, Lachenbruch PA (2004) Fatalities caused by TRALI. Transfus Med Rev 18:184–188
Kalhan R, Mikkelsen M, Dedhiya P, Christie J, Gaughan C, Lanken PN, Finkel B, Gallop R, Fuchs BD (2006) Underuse of lung protective ventilation: analysis of potential factors to explain physician behavior. Crit Care Med 34:300–306
Kelher MR, Masuno T, Moore EE, Damle S, Meng X, Song Y, Liang X, Niedzinski J, Geier SS, Khan SY, Gamboni-Robertson F, Silliman CC (2009) Plasma from stored packed red blood cells and MHC class I antibodies causes acute lung injury in a 2-event in vivo rat model. Blood 113:2079–2087
Kessels LW, Visser OJ (2005) Acute dyspnoea following transfusion of plasma-containing blood products. Ned Tijdschr Geneeskd 149:369–371
Leach M, Vora AJ, Jones DA, Lucas G (1998) Transfusion-related acute lung injury (TRALI) following autologous stem cell transplant for relapsed acute myeloid leukaemia: a case report and review of the literature. Transfus Med 8:333–337
Leger R, Palm S, Wulf H, Vosberg A, Neppert J (1999) Transfusion-related lung injury with leukopenic reaction caused by fresh frozen plasma containing anti-NB1. Anesthesiology 91:1529–1532
Looney MR, Nguyen JX, Hu Y, Van Ziffle JA, Lowell CA, Matthay MA (2009) Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. J Clin Invest 119:3450–3461
Looney MR, Su X, Van Ziffle JA, Lowell CA, Matthay MA (2006) Neutrophils and their Fcgamma receptors are essential in a mouse model of transfusion-related acute lung injury. J Clin Invest 116:1615–1623
Lydaki E, Bolonaki E, Nikoloudi E, Chalkiadakis E, Iniotaki-Theodoraki A (2005) HLA class II antibodies in transfusion-related acute lung injury (TRALI). A case report. Transfus Apher Sci 33:107–111
Moore SB (2006) Transfusion-related acute lung injury (TRALI): clinical presentation, treatment, and prognosis. Crit Care Med 34:S114–S117
Rana R, Fernandez-Perez ER, Khan SA, Rana S, Winters JL, Lesnick TG, Moore SB, Gajic O (2006) Transfusion-related acute lung injury and pulmonary edema in critically ill patients: a retrospective study. Transfusion 46:1478–1483
Silliman CC (2006) The two-event model of transfusion-related acute lung injury. Crit Care Med 34:S124–S131
Silliman CC, Bjornsen AJ, Wyman TH, Kelher M, Allard J, Bieber S, Voelkel NF (2003) Plasma and lipids from stored platelets cause acute lung injury in an animal model. Transfusion 43:633–640
Silliman CC, Voelkel NF, Allard JD, Elzi DJ, Tuder RM, Johnson JL, Ambruso DR (1998) Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. J Clin Invest 101:1458–1467
Stainsby D, Jones H, Asher D, Atterbury C, Boncinelli A, Brant L, Chapman CE, Davison K, Gerrard R, Gray A, Knowles S, Love EM, Milkins C, McClelland DB, Norfolk DR, Soldan K, Taylor C, Revill J, Williamson LM, Cohen H (2006) Serious hazards of transfusion: a decade of hemovigilance in the UK. Transfus Med Rev 20:273–282
Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS (1997) Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest 99:944–952
Vaneker M, Halbertsma FJ, van EJ, Netea MG, Dijkman HB, Snijdelaar DG, Joosten LA, van der Hoeven JG, Scheffer GJ (2007) Mechanical ventilation in healthy mice induces reversible pulmonary and systemic cytokine elevation with preserved alveolar integrity: an in vivo model using clinical relevant ventilation settings. Anesthesiology 107:419–426
Vlaar AP, Binnekade J, Prins D, van Stein D, Schultz M, Juffermans N (2010) Risk factors and outcome of transfusion-related acute lung injury in the critically ill—a nested case control study. Crit Care Med 38:771–778
Vlaar AP, Binnekade JM, Schultz MJ, Juffermans NP, Koopman MM (2008) Preventing TRALI: ladies first, what follows? Crit Care Med 36:3283–3284
Vlaar AP, Schultz MJ, Juffermans NP (2009) Transfusion-related acute lung injury: a change of perspective. Neth J Med 67:320–326
Vlaar AP, Wortel K, Binnekade JM, van Oers MH, Beckers E, Gajic O, Schultz MJ, Juffermans NP (2009) The practice of reporting transfusion-related acute lung injury: a national survey among clinical and preclinical disciplines. Transfusion 50:443–451
Wallis JP, Lubenko A, Wells AW, Chapman CE (2003) Single hospital experience of TRALI. Transfusion 43:1053–1059
Wiersum-Osselton JC, Porcelijn L, van Stein D, Vlaar AP, Beckers EA, Schipperus MR (2008) Transfusion-related acute lung injury (TRALI) in the Netherlands in 2002–2005. Ned Tijdschr Geneeskd 152:1784–1788
Williamson LM, Lowe S, Love EM, Cohen H, Soldan K, McClelland DB, Skacel P, Barbara JA (1999) Serious hazards of transfusion (SHOT) initiative: analysis of the first two annual reports. BMJ 319:16–19
Wolthuis EK, Choi G, Dessing MC, Bresser P, Lutter R, Dzoljic M, van der PT, Vroom MB, Hollmann M, Schultz MJ (2008) Mechanical ventilation with lower tidal volumes and positive end-expiratory pressure prevents pulmonary inflammation in patients without preexisting lung injury. Anesthesiology 108:46–54
Wolthuis EK, Vlaar AP, Choi G, Roelofs JJ, Haitsma JJ, van der Poll T, Juffermans NP, Zweers MM, Schultz MJ (2009) Recombinant human soluble tumor necrosis factor-alpha receptor fusion protein partly attenuates ventilator-induced lung injury. Shock 31:262–266
Wolthuis EK, Vlaar AP, Choi G, Roelofs JJ, Juffermans NP, Schultz MJ (2009) Mechanical ventilation using non-injurious ventilation settings causes lung injury in the absence of pre-existing lung injury in healthy mice. Crit Care 13:R1
Wright SE, Snowden CP, Athey SC, Leaver AA, Clarkson JM, Chapman CE, Roberts DR, Wallis JP (2008) Acute lung injury after ruptured abdominal aortic aneurysm repair: the effect of excluding donations from females from the production of fresh frozen plasma. Crit Care Med 36:1796–1802
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
134_2010_1802_MOESM2_ESM.ppt
Flow diagram of the study design. The mice (n=84) were randomly assigned to 7 groups (n=12 per group). Three groups of animals (infusion of PBS, infusion of isotype antibody, infusion of MHC-I class antibody) served as non-ventilated controls and were sacrificed after 2 h. The other animals were mechanically ventilated with two different strategies (Low VT (mice ventilated for 5 h with a tidal of 7.5 ml/kg) or High VT (mice ventilated for 5 h with a tidal of 15 ml/kg)) and received either PBS infusion or MHC-I class infusion after 3 h of ventilation (PPT 31 kb)
134_2010_1802_MOESM3_ESM.doc
Respiratory rate of non-ventilated mice receiving PBS infusion, isotype control antibody or MHC-I antibody. Infusion of MHC-I antibodies resulted in respiratory distress as measured by an increase in respiratory rate, compared to the PBS and isotype controls. In the MHC-I antibody group, 40% of the mice showed respiratory failure which resulted in death after 2 h (data not shown). *P<0.01 (DOC 20 kb)
Rights and permissions
About this article
Cite this article
Vlaar, A.P.J., Wolthuis, E.K., Hofstra, J.J. et al. Mechanical ventilation aggravates transfusion-related acute lung injury induced by MHC-I class antibodies. Intensive Care Med 36, 879–887 (2010). https://doi.org/10.1007/s00134-010-1802-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-1802-z