Abstract
Purpose
Probiotics have been shown to be able to restore a non-pathogenic digestive flora, to prevent digestive colonization by pathogenic bacteria, and to modulate immunity. The aim of this study was to assess the effects of prophylactic probiotic administration in patients ventilated for up to 2 days.
Methods
This study was performed as a double-blind, concealed randomized, placebo-controlled trial in a French medical intensive care unit (ICU). Adult patients mechanically ventilated for a period of more than 48 h received enterally administered probiotics (Ergyphilus ®, 2 × 1010 lactic acid bacteria, mostly Lactobacillus rhamnosus GG, once a day) or placebo until successful weaning.
Results
A total of 167 patients were included. The two groups were comparable at baseline. The 28-day mortality rates were not different in the probiotic (25.3%) and placebo groups (23.7%). Mortality rates in ICU and at 90 days were also unaffected by the treatment. The incidence of ICU-acquired infections did not differ significantly except for that of catheter-related bloodstream infections that was lowered by probiotics. On a prespecified subgroup analysis, we found a reduction of the 28-day mortality among severe sepsis patients (total n = 101) treated with probiotics (n = 52) with an odds ratio (OR) for death at 0.38 (95% CI 0.16–0.93, p = 0.035). By contrast, probiotics were associated with a higher mortality rate in non-severe sepsis patients (OR 3.09, 95% CI 0.87–11.01, p = 0.08).
Conclusions
Although numerous uncertainties remain (type and the number of strains to use, delay and length of administration), and despite an acceptable safety profile, the daily prophylactic administration of probiotics cannot be encouraged in the critically ill patient.
Similar content being viewed by others
Introduction
Healthcare-associated infections (HAI) constitute a major focus of public health because of their consequences in terms of mortality, morbidity, and financial costs. Owing to multiple pathophysiological factors, such as immune dysfunction or digestive barrier dysfunction, the critically ill patient is indeed particularly prone to HAI, often after an early colonization of the digestive tube by resistant bacteria [1]. Among these infections, ventilator-associated pneumonia (VAP), with an incidence around 15% and an attributable mortality reaching 50% in some studies, is a daily problem for intensivists [2].
Prevention of colonization by resistant bacteria is one of the numerous approaches to fight HAI. Strategies based on the prophylactic prescription of antibiotics (as selective digestive decontamination) could be proposed in this context. However, in this era of growing bacterial resistances, and of relative exhaustion of the antibiotic pipeline, emergence of new “non-antibiotics” strategies such as administration of probiotics could be interesting [3, 4].
First observations made by Elie Metchnikoff one century ago of the greater longevity of Bulgarian peasants submitted to a diet including yoghurts containing some lactic acid bacterias (LABs) have constituted the foundations of the concept of probiotics, reintroduced 60 years later by Lilly and Stillwell [5]. Probiotics are defined by the World Health Organization and the Food and Agriculture Organization as “viable microorganisms that, when ingested in a sufficient amount, can be beneficial for health” [6]. They are now available in different forms to the public and are the subject of a flourishing trade in western countries.
On the basis of growing and convincing experimental evidence the use of probiotics has extended to a wide variety of inflammatory digestive or extra-digestive diseases. Probiotics, by restoring non-pathogenic digestive flora, preventing digestive colonization by pathogenic bacteria, modulating local and general immunity, and decreasing intestinal hyperpermeability [7], could be prove beneficial in the critically ill patient, though the experience of using these products in the ICU is still limited. To date only nine studies evaluated the effects of probiotics administration in the critically ill patient and conclusions are difficult to draw because of the heterogeneity of the clinical situations and of the strains used [8]. Furthermore, none of these studies had mortality as a primary endpoint.
We therefore conducted a prospective trial to evaluate the effects of probiotics on the outcome of mechanically ventilated critically ill patients.
The results of this study were partly presented at the national congress of the Société de Réanimation de Langue Française in January 2009.
Patients and methods
Study design
This was a randomized, double-blind, placebo-controlled trial of prophylactic probiotics administration in patients mechanically ventilated for up to 2 days. This study was sponsored by the University Hospital of Nancy and is registered on clinicaltrial.gov (identifier NCT00122408). The protocol was approved by local institutional review board and by the Comité de Protection des Personnes Est III. Primary endpoint was 28-day mortality. Secondary endpoints were mortality at day 90, reversal of organ failure, occurrence of ICU-acquired infections and colonization by day 28, and ICU length of stay.
Studied population
All intubated adult patients under mechanical ventilation for a predicted period of at least 2 days were eligible.
Exclusion criteria were (1) a predicted duration of mechanical ventilation less than 2 days, (2) age less than 18 years, (3) pregnancy, (4) immunosuppression (AIDS, malignant hemopathy, neutrophil count less than 500 per mm3, cytostatic chemotherapy during past 3 months before ICU admission), (5) short bowel disease (a situation known to be a risk factor for development of LAB-associated infections), and (5) inclusion in another trial. Patients re-admitted to the ICU and previously included in this study could not be randomized again.
Patients were enrolled as soon as possible following their admission and/or intubation after the obtainment of written informed consent (from patient, relative, or legal representative).
Procedures
After inclusion, eligible patients were randomly assigned by the pharmacist in a 1:1 ratio to the probiotics or the placebo group by using a concealed randomization table provided by the Service d’épidémiologie et d’évaluation cliniques. A block size of 4 was used without further stratification. Treatment consisted of the administration of either 5 Ergyphilus® (Nutergia, Capdenac, France) or placebo capsules once a day. Ergyphilus® capsules consisted in a multispecies probiotic preparation containing 2 × 1010 of revivable bacteria (mainly Lactobacillus rhamnosus GG, but also Lactobacillus casei, Lactobacillus acidophilus, and Bifidobacterium bifidum) whereas placebo capsules only contained the excipient. Both preparations were identical in appearance, weight, odor, taste, consistency, and packaging. Treatments were diluted in 20 mL of water and administered daily by the nurse through the enteral feeding tube for the entire period of mechanical ventilation (but for a duration not exceeding 28 days). After weaning from the ventilator, treatment was given for two additional days and then stopped in the case of successful extubation, or continued in the case of extubation failure. All patients received enteral nutrition (Fresubin®, Fresenius Kabi, Homburg, Germany) within 24 h of admission starting at 10 kcal/kg and then progressively rising to 30–35 kcal/kg through nasogastric tube.
Usual demographic, clinical, and biological data, as well as the occurrence of adverse events were collected. ICU-acquired infections (bloodstream and catheter-related infections, ventilator-associated pneumonia, urinary tract infection) [9] were prospectively recorded by the physicians in charge of the patient. Specifically VAP was defined by the presence of (a) a new and persistent infiltrate on chest radiograph associated with at least one of the following: purulent tracheal secretions, temperature 38.3°C or higher, and a leukocyte count of 10,000 μL−1 or higher; and (b) positive quantitative cultures of distal pulmonary secretions obtained from bronchoalveolar lavage (significant threshold more than 104 colony-forming units/mL). Catheter-related bloodstream infection (CR-BSI) was defined as a positive peripheral blood culture, regardless of the microorganism, associated with a positive catheter-tip culture growing the same microorganism as in the blood. Nasal and rectal colonization with multi-drug resistant (MDR) bacteria were also recorded, as well as diarrhoea episodes defined by the occurrence of at least 3 liquid stools/day [10].
Statistical analysis
We calculated that 740 patients would be required in order to demonstrate a treatment effect on 28-day mortality (from 25 to 20%), with a 90% power at α risk of 0.05. A blinded interim analysis was planned after randomization of 200 patients. Because of the worrying conclusions of the Besselink’ study [11] an unplanned interim analysis was performed after the inclusion of the first 167 patients to verify the absence of serious adverse events or abnormally high death rates. An independent data and safety monitoring committee evaluated this unplanned interim analysis. An O’Brien–Fleming approach was used for sequential stopping rules for safety and efficacy according to the Lan–DeMets method [12]. After the analysis, the data and safety monitoring committee recommended that the study be stopped for futility as the corrected lower boundary was crossed: it was calculated that more than 4,000 patients per group would be necessary to objectively establish a treatment effect.
All data analyses were done in accordance with a pre-established analysis plan. The incidence of the primary endpoint (28-day mortality) and secondary endpoints were compared between the groups and results are presented as relative risk with 95% CI. The Kolmogorov–Smirnov test was used to assess whether continuous data were normally distributed. For continuous variables, differences between groups were tested with Student’s t test for normally distributed data or Mann–Whitney U test for non-normally distributed data. Kaplan–Meier curves with log-rank tests were generated. All analyses were done on the basis of the intention-to-treat principle. Pre-specified subgroup analyses [severe sepsis and septic shock patients (this subgroup is henceforth referred to as severe sepsis patients) [13], and delay to treatment less than 48 h after admission] were performed by using logistic regression models to do a formal test for interaction to assess whether treatment effects differed significantly between these subgroups. A two-sided p value less than 0.05 was deemed statistically significant. All statistical analyses were done with SAS software (version 12).
Results
Characteristics of the patients
Eligibility was determined for a total of 845 ICU admissions between February 2006 and March 2008. Among eligible patients, 167 were enrolled (Fig. 1). Non-eligible patients had a short ICU stay, and were most often admitted because of metabolic disorders, or suicide attempts.
There were no differences in baseline characteristics between patients in the placebo group and those in the probiotic group (Table 1).
Probiotic or placebo treatment was administered within 2.42 ± 1.84 days after ICU admission and according to the protocol on 88.75 and 89.70% of all the study’s days, respectively (p = not significant, n.s.). Protocol violation was most often due to an omission of the nurse in charge of the patient.
Intention to treat and per protocol analyses yielded similar results and thus only the former is shown here.
Primary and secondary endpoints
Treatment effects on primary and secondary endpoints are depicted on Table 2.
Overall, 28-day crude mortality rate was 24.5%. Mortality rate at day 28 was 25.3% in the probiotic group, not significantly different from that observed in the placebo group (23.7%, p = 0.80). On a random effect logistic regression model adjusted for age, sex, the simplified acute physiology score II (SAPSII), McCabe [14], and sequential organ failure assessment (SOFA) scores at admission, the odds ratio (OR) for death during the first 28 days for the probiotic group as compared to the placebo group was 0.89 (95% CI 0.45–1.75, p = 0.73). There was no effect of treatment on the 90-day mortality rate (31.0 vs. 30.0% for probiotic and placebo groups, respectively, p = 0.90). A Kaplan–Meier survival curve (Fig. 2) confirmed this absence of treatment effect (log-rank test, p = 0.91).
There was no effect of probiotics administration on ICU and hospital lengths of stay or organ failure resolution (Table 2).
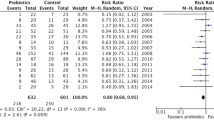
From a microbiological standpoint, an unexpected decline of catheter-related bloodstream infections (CR-BSI) was noticed in the probiotic group (1.84 vs. 6.78‰ catheter-days in the probiotic and placebo groups, respectively, p = 0.005). No significant alteration was conferred by probiotics on urinary tract infections, ventilator-associated pneumonia, or on a composite infectious criteria (combination of all ICU-acquired infections) (Table 2). When only looking at infections occurring after 96 h of ICU stay, results were similar (Table 2). A logistic regression model confirmed these findings (Fig. 3) with an OR for ICU-acquired infections of 0.87 (95% CI 0.46–1.65, p = 0.68). Furthermore, probiotic treatment did not decrease multi-drug resistant bacteria nasal (n = 4 vs. 4 patients) and/or rectal colonization (n = 11 vs. 12 patients), and did not reduce antibiotic consumption (Table 2).
Subgroup analysis: severe sepsis patients
On a predefined subgroup analysis (Tables 1 and 2 in the “Electronic supplementary material”), we found a 28-day mortality reduction among severe sepsis patients (total n = 101) treated with probiotics (n = 52) with an OR for death at 0.38 (95% CI 0.16–0.93, p = 0.035, Fig. 3). At 90 days, mortality rates in probiotic and placebo groups were 25 and 41%, respectively, and hazard ratio for 90-day deaths was 0.52 (95% CI 0.26–1.04). These trends were confirmed by the Kaplan–Meier survival curve (Fig. 4).
By contrast, among the severe sepsis patients, probiotics administration showed no effect on ICU-acquired infections incidence or ICU and hospital lengths of stay.
Subgroup analysis: non-severe sepsis patients
When looking at the non-severe sepsis subgroup, patients who received probiotics had a higher mortality at 28 and 90 days than placebo-treated patients (Supplementary Table 2). This fact was confirmed by a survival analysis (Supplementary Figure): the hazard ratio for 90-day death was 3.40 (95% CI 1.18–7.64, p = 0.02).
Early treatment administration (within the 2 first days after ICU admission) was not superior to delayed administration on primary and secondary outcomes (Fig. 3).
Safety analysis
There were no side effects, severe or not, observed during the study period. Specifically, there were no bacteremias due to LAB and no bowel ischemia.
Discussion
Critically ill patients are at very high risk of developing HAI that may lead to multiple organ failure and impair prognosis. Several experimental and clinical studies suggest that an inexpensive and easy to use treatment, namely enteral probiotics administration, could be effective in the ICU by improving immune competency, reducing oropharyngeal bacterial colonization, and even nosocomial infections [1–4].
To our knowledge, this is the first randomized, controlled trial in ICU patients that investigates the effects of probiotics administration with 28-day mortality as a primary endpoint.
The main result of this study is that the administration of probiotics has no impact on mortality among patients mechanically ventilated for more than 2 days. This result seems in accordance with previous reports by Jain, McNaught, and Kotzampassi [15–17], respectively, performed in 90, 103, and 65 surgical ICU patients, who found no effect of probiotics on mortality, evaluated as a secondary endpoint. On a pre-planned subgroup analysis, we observed a significant mortality reduction among the septic patients treated with probiotics (OR 0.38 [95% CI 0.16–0.93], p = 0.035). Although this finding may be explained by the action of probiotics on immunity (immune stimulation), there was no effect on the incidence of ICU-acquired infections in this subgroup. Another explanation may come from the fact that septic patients were sicker than non-septic ones and a treatment effect may have been only apparent in the more severe patients. Nevertheless, our data must be viewed as hypothesis-generating findings and should be confirmed by additional specific studies. By contrast, when probiotic treatment was administered to non-severe sepsis patients, it was associated with a higher risk of death. This finding can simply be explained by the unexpectedly low mortality of placebo-treated patients (13% at day 90) despite the fact that 30% of them suffered from a shock and presented with a high SOFA score (8.0 ± 4.5). But we cannot exclude a real deleterious effect of probiotics on these less critically ill patients than those included in the severe sepsis subgroup, although neither was linked to superinfections or LAB bacteraemia. Indeed, it has recently been shown that deleterious effects of probiotics are only expected to appear among the more severe patients, though we observed the opposite here [18]. Although we have no obvious explanation to reconcile these different findings, we can just make the hypothesis that probiotics administration should have induced an inappropriate innate immune activation in the less severe group of patients.
A second important result of the current study is that probiotics were not associated with deleterious side effects, such as those reported by Besselink et al. [11], e.g., bowel ischemia and death. Although reassuring, it must be underlined that only one patient with acute pancreatitis was included in our trial (probiotic group: uncomplicated evolution): therefore, these two studies are not comparable in terms of case-mix. Other differences between the current study and the Besselink’s one do exist: (1) probiotics strains differed, and (2) our patients received enteral nutrition in the stomach and not in their jejunum.
Regarding the secondary endpoint “ICU-acquired infections”, we observed that probiotics prophylaxis was associated with a decreased incidence of catheter-related bloodstream infection (from 6.78 to 1.84‰ catheter-days), though the incidence of other types of nosocomial infections, and especially ventilator-associated pneumonia, was not reduced. This surprising finding must be tempered by the fact that CR-BSI incidence was unexpectedly high in the control group; therefore, it may be that probiotics have no effect when dealing with lower CR-BSI incidences. Data on the probiotics effects on the incidence of HAI are conflicting. Although Kotzampassi et al., and Spindler-Vesel et al. (113 trauma patients) observed a 25% decrease of HAI and VAP in probiotics-treated patients [17, 19], our findings seem to be in agreement with those of Forestier et al. (medical ICU patients) [20] and Besselink et al. [11], who were unable to observe any reduction of nosocomial infections.
One of the alleged mechanisms of action of probiotics is the prevention of bacterial digestive and extra-digestive colonization: here we did not find any effect of probiotics in terms of colonization rates (rectal and nasal) or timing of colonization with MDR bacteria.
Probiotics are now considered as dietary supplements in western countries, and their marketing, if not completely unregulated, is not submitted to the same regulation and safety monitoring as medical drugs. Side effects such as bloodstream infections, endocarditis, abscess, and even pneumonia due to probiotic LAB, have thereby been progressively described in the literature, mostly in immunosuppressed or short bowel disease-affected patients [1–4]. Here we did not observe any serious adverse event linked to probiotics administration despite the fact that a certain degree of ‘immune paralysis’ must have been present in our patients (many of them suffering from septic shock).
The principal limitation of this study stems from the fact that it has been prematurely stopped. Indeed, we planned an interim analysis after the first 200 patients were included. Before we reached this milestone, the study from Besselink et al. [11] was released showing elevated death rates in the probiotic group. For safety’s sake, we thus decided to perform the interim analysis shortly after publication of the aforementioned article. As no global trend, even minor, emerged at this stage, increasing the population size will probably not be able to bring further information.
Conclusion
We were unable to observe protective effects of the use of probiotics in critically ill patients ventilated for up to 2 days. Although numerous uncertainties remain (type and the number of strains to be used, delay and length of administration), and despite an acceptable safety profile, especially the absence of associated bowel ischemia, the daily prophylactic administration of probiotics cannot be encouraged in the critically ill patient, notably in non-severe sepsis patients.
References
Isakow W, Morrow LE, Kollef MH (2007) Probiotics for preventing and treating nosocomial infections: review of current evidence and recommendations. Chest 132:286–294
McNabb B, Isakow W (2008) Probiotics for the prevention of nosocomial pneumonia: current evidence and opinions. Curr Opin Pulm Med 14:168–175
Watkinson PJ, Barber VS, Dark P, Young JD (2007) The use of pre- pro- and synbiotics in adult intensive care unit patients: systematic review. Clin Nutr 26:182–192
Saavedra JM (2001) Clinical applications of probiotic agents. Am J Clin Nutr 73:1147S–1151S
Lilly DM, Stillwell RH (1965) Probiotics: growth-promoting factors produced by microorganisms. Science 147:747–748
Guidelines for the evaluation of probiotics in food: report of a joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food (2002) London, ON, Canada
Walker WA (2002) Mechanisms of action of probiotics. Clin Infect Dis 46(Suppl 2):S87–S91
Siempos II, Ntaidou TK, Falagas ME (2010) Impact of the administration of probiotics on the incidence of ventilator-associated pneumonia: a meta-analysis of randomized controlled trials. Crit Care Med 38:954–962
Calandra T, Cohen J, International Sepsis Forum Definition of Infection in the ICU Consensus Conference (2005) The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med 33:1538–1548
Whelan K, Judd PA, Taylor MA (2003) Defining and reporting diarrhoea during enteral tube feeding: do health professionals agree? J Hum Nutr Diet 16:21–26
Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, Nieuwenhuijs VB, Bollen TL, van Ramshorst B, Witteman BJ, Rosman C, Ploeg RJ, Brink MA, Schaapherder AF, Dejong CH, Wahab PJ, van Laarhoven CJ, van der Harst E, van Eijck CH, Cuesta MA, Akkermans LM, Gooszen HG, Dutch Acute Pancreatitis Study Group (2008) Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 371:651–659
Lan KKG, DeMets DL (1983) Discrete sequential boundaries for clinical trials. Biometrika 70:659–663
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101:1644–1655
McCabe WR, Jackson GG (1962) Gram negative bacteremia: I. Etiology and ecology. Arch Intern Med 110:845–847
Jain PK, McNaught CE, Anderson AD, MacFie J, Mitchell CJ (2004) Influence of synbiotic containing Lactobacillus acidophilus La5, Bifidobacterium lactis Bb 12, Streptococcus thermophilus, Lactobacillus bulgaricus and oligofructose on gut barrier function and sepsis in critically ill patients: a randomised controlled trial. Clin Nutr 23:467–475
McNaught CE, Woodcock NP, Anderson AD, MacFie J (2005) A prospective randomised trial of probiotics in critically ill patients. Clin Nutr 24:211–219
Kotzampassi K, Giamarellos-Bourboulis EJ, Voudouris A, Kazamias P, Eleftheriadis E (2006) Benefits of a synbiotic formula (Synbiotic 2000Forte) in critically Ill trauma patients: early results of a randomized controlled trial. World J Surg 30:1848–1855
Besselink MG, van Santvoort HC, Renooij W, de Smet MB, Boermeester MA, Fischer K, Timmerman HM, Ahmed Ali U, Cirkel GA, Bollen TL, van Ramshorst B, Schaapherder AF, Witteman BJ, Ploeg RJ, van Goor H, van Laarhoven CJ, Tan AC, Brink MA, van der Harst E, Wahab PJ, van Eijck CH, Dejong CH, van Erpecum KJ, Akkermans LM, Gooszen HG, Dutch Acute Pancreatitis Study Group (2009) Intestinal barrier dysfunction in a randomized trial of a specific probiotic composition in acute pancreatitis. Ann Surg 250:712–719
Spindler-Vesel A, Bengmark S, Vovk I, Cerovic O, Kompan L (2007) Synbiotics, prebiotics, glutamine, or peptide in early enteral nutrition: a randomized study in trauma patients. J Parenter Enteral Nutr 31:119–126
Forestier C, Guelon D, Cluytens V, Guillart T, Sirot J, De champs C (2008) Oral probiotic and prevention of Pseudomonas aeruginosa infections: a randomized, double-blind, placebo-controlled pilot study in intensive care unit patients. Crit Care 12:R69
Acknowledgment
This work was financially funded by CHU Nancy.
Conflict of interest
None of the authors declare any conflict of interest in relation to this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Barraud, D., Blard, C., Hein, F. et al. Probiotics in the critically ill patient: a double blind, randomized, placebo-controlled trial. Intensive Care Med 36, 1540–1547 (2010). https://doi.org/10.1007/s00134-010-1927-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-1927-0