Abstract
Purpose
To compare characteristics, clinical evolution and outcome in adult patients with influenza A (H1N1) acute respiratory distress syndrome (ARDS) treated with or without extracorporeal membrane oxygenation (ECMO).
Methods
A prospective observational study of patients treated in Marseille South Hospital from October 2009 to January 2010 for confirmed influenza A (H1N1)-related ARDS. Clinical features, pulmonary dysfunction and mortality were compared between patients treated with and without ECMO.
Results
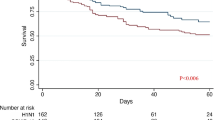
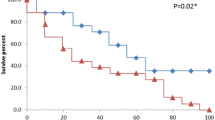
Of 18 patients admitted, 6 were treated with veno-venous and 3 with veno-arterial ECMO after median (interquartile, IQR) duration of mechanical ventilation of 10 (6–96) h. Six ECMO were initiated in a referral hospital by a mobile team, a median (IQR) of 3 (2–4) h after phone contact. Before ECMO, patients had severe respiratory failure with median (IQR) PaO2 to FiO2 ratio of 52 (50–60) mmHg and PaCO2 of 85 (69–91) mmHg. Patients treated with or without ECMO had the same hospital mortality rate (56%, 5/9). Duration of ECMO therapy was 9 (4–14) days in survivors and 5 (2–25) days in non-survivors. Early improvement of PaO2 to FiO2 ratio was greater in ECMO survivors than non-survivors after ECMO initiation [295 (151–439) versus 131 (106–144) mmHg, p < 0.05]. Haemorrhagic complications occurred in four patients under ECMO, but none required surgical treatment.
Conclusions
ECMO may be an effective salvage treatment for patients with influenza A (H1N1)-related ARDS presenting rapid refractory respiratory failure, particularly when provided by a mobile team allowing early cannulation prior to transfer to a reference centre.
Similar content being viewed by others
Introduction
Extracorporeal membrane oxygenation (ECMO) uses cardiopulmonary bypass technology to assist gas exchange in acute respiratory distress syndrome (ARDS) patients, allowing ventilator settings to be reduced, thus providing time for treatment and recovery. It has been provided in specialized centres as an alternative therapy for patients responding poorly to conventional mechanical ventilation. Until recently, use of ECMO remained controversial because of negative results reported in previous randomized studies [1, 2]. However, over the last two decades, the technique itself and ventilator management have progressed considerably, and several studies have reported encouraging survival rates [3, 4]. Indeed, recently an ECMO-based management protocol for selected ARDS patients transferred to a reference centre was shown to improve 6-month disability-free survival [5]. From 2009, the influenza A (H1N1) worldwide pandemic has been responsible for severe cases of pneumonia and ARDS [6–10]. In Canada, only six patients (3.6%) were treated with ECMO for influenza H1N1-related ARDS, with a mortality rate of 33% [11]. Conversely, use of ECMO was more frequent in Southern Hemisphere intensive care units (ICUs), with 68 of 201 (33.8%) mechanically ventilated influenza H1N1 patients treated with ECMO. The corresponding mortality rate was 21% [8].
Marseille South Hospital is a general acute-care, university hospital. It is a reference centre for treatment of severe respiratory infections. Since we expected a high rate of severe ARDS due to influenza H1N1 requiring ECMO therapy based on the data from the Southern Hemisphere [8], we established an ECMO-based protocol in autumn 2009. This protocol included a mobile unit that could initiate ECMO in referral hospitals of our region before transfer to our centre.
This study compared epidemiological characteristics, clinical features and outcome of adult patients with confirmed influenza H1N1-related ARDS treated in our centre with or without ECMO.
Methods
We included all adult patients with influenza H1N1-related ARDS admitted initially or secondarily to our ICU. Positive rapid influenza diagnostic test results were confirmed using quantitative real-time polymerase chain reaction (PCR) assays on nasal swabs. The protocol was approved by the local research ethics committee, who waived the need for informed consent, according to French legislation.
Prospectively collected data included demographic data, presence of identified risk factors for severe influenza H1N1 pneumonia and major co-morbidities, respiratory and haemodynamic parameters at admission, before ECMO initiation and throughout ARDS evolution, technical characteristics of ECMO therapy, complications and outcome. Severity of illness was assessed using the Murray Lung Injury Score [12], the Simplified Acute Physiology Score (SAPS) II [13] at ICU admission and the Sequential Organ Failure Assessment (SOFA) [14] score before ECMO initiation or over the 6 h following initiation of mechanical ventilation in patients not receiving ECMO.
ECMO-based management protocol
Before consideration for ECMO, patients were sedated using continuous neuromuscular blockade and ventilated with volume-controlled ventilation using tidal volume (Vt) of 5–7 ml/kg predicted body weight and positive end-expiratory pressure (PEEP) level ≥10 cmH2O. ECMO therapy was indicated if patients presented PaO2 to FiO2 ratio of less than 70 mmHg for at least 2 h under FiO2 of 1 and PEEP level adjusted to obtain a plateau pressure (Pplat) of 30 cmH2O, or PaO2 to FiO2 ratio of less than 100 mmHg associated with Pplat >35 cmH2O, or respiratory acidosis with pH ≤7.15 despite respiratory rate ≥35/min. Absolute contraindications included: any contraindication to heparin treatment, chronic disease expected to be fatal within 5 years, Sequential Organ Failure Assessment (SOFA) score >16 or age >70 years. Relative contraindications were: body mass index (BMI) >40 kg/m2 and duration of ARDS ≥7 days.
ECMO was initiated in our ICU, or in the admitting referral hospital located in our region (31,400 km2 area). In the latter case, the patient was transferred to our ICU immediately after ECMO initiation by a team comprising: one intensivist from our ICU, the cardiac surgeon who cannulated the patient and a perfusionist. Veno-venous ECMO was instituted using surgical cannulation. However, echocardiography was performed in all patients before cannulation and each day under ECMO. This allowed veno-arterial cannulation to be performed initially or later in patients presenting echographic left heart failure, defined as <30% left ventricular ejection fraction. The surgical approach was chosen because of the experience and availability of cardiac surgeons in our institution. We used centrifugal pumps (Bio-console 560; Medtronic Perfusion Systems, Minneapolis, MN, USA) with flow of 3–5 l/min in all patients. Circuits were heparin-coated and composed of Quadrox D with Bioline Coating oxygenators (Maquet, Hirrlingen, Germany), 20 Fr cannulae (Edwards Lifesciences, Irvine, CA, USA) and Intersept polyvinyl chloride (PVC) class VI tubing (Medtronic). Initial ventilator settings were: Pplat 20–30 cmH2O, PEEP 15–20 cmH2O, respiratory rate 5–10/min and FiO2 adjusted to obtain arterial O2 saturation of 90–95% while FiO2 was set at 1 on the oxygenator. Continuous heparin infusion maintained activated partial thromboplastin time (APTT) at 40–50 s. Triggering limits for transfusion were 80 × 103/ml for platelets and 10 g/dl for haemoglobin. ECMO was managed by the ICU team with daily intermittent perfusionist support. It was continued until lung recovery, or until irreversible multiorgan failure. Patients were weaned from veno-venous ECMO when the following criteria were met: PaO2 to FiO2 ratio >200 mmHg with PEEP ≤12 cmH2O, Pplat <32 cmH2O with Vt 5–7 ml/kg, with FiO2 of 0.21 on ECMO, blood flow of 1 l/min and gas flow of 1 l/min.
Analysis
Descriptive statistics included frequency analysis (percentages) for categorical variables and medians and interquartile ranges (IQR) for continuous variables. Comparisons used the Fisher exact test for categorical variables and Mann–Whitney U test for continuous variables. Statistical analysis was performed using SPSS 12.0 software (SPSS, Chicago, IL, USA).
Results
Patient characteristics
Between October 2009 and January 2010, 139 patients were admitted to our institution for confirmed influenza A (H1N1). Among the 22 patients requiring mechanical ventilation, 18 were admitted to our ICU for ARDS. No cases of bacterial superinfection were diagnosed on admission. Among the ten patients who met the criteria for ECMO, nine received it whereas one patient died of cardiac arrest due to hypoxaemia before ECMO could be organized.
Table 1 summarises patient characteristics. Early-stage chronic lymphocytic leukaemia was detected in two patients receiving ECMO. Of these, one died and one was diagnosed during ICU stay. Of the patients not receiving ECMO, two suffered from cancer (end-stage sarcoma and chronic lymphocytic leukaemia); both died. Compared with patients treated without ECMO, those receiving ECMO presented more often with shock requiring vasopressors (7/9 patients versus 2/9, p = 0.05); they also had higher median (IQR) lactate levels [4.9 (1.9–12.6) versus 1.6 (1.4–2) mmol/l, p < 0.05].
All patients treated with ECMO had refractory hypoxaemia (Table 2) associated with respiratory acidosis (four had pH <7.15). Prior to ECMO, prone position was used in two patients, and nitric oxide in six. Two patients treated with ECMO had BMI >40 kg/m2, and one patient required emergency Caesarean before ECMO initiation.
Details and effects of ECMO support
Median (IQR) duration of mechanical ventilation before ECMO therapy was 10 (6–96) h. Cannulation was performed in a referral hospital by the mobile unit for six patients. Median (IQR) time between contact from the referral hospital and patient cannulation was 3 (2–4) h; transfer to our centre was within 7 (3–8) h. No complication was noted during transfer. Six patients were treated with veno-venous ECMO and three with initial veno-arterial cannulation. Vascular cannulae were inserted through femoral veins or arteries. Median (IQR) ECMO flow was 4.2 (3.7–4.6) l/min on day 1, 3.3 (3.1–3.6) on day 2 and 3.8 (3.6–4) on day 3. Median (IQR) heparin dosage infused during ECMO therapy was 9 (7–11) UI/kg/h. ECMO associated with protective ventilation rapidly decreased PaCO2 in all patients. In contrast, improvement in PaO2 was more marked in survivors, despite similar ventilator parameters (Table 3) and similar ECMO settings [pump flow 4 (3.7–4.5) l/min in survivors on first day versus 4.2 (3.4–4.6) in non-survivors, FiO2 = 1]. Decreasing vasopressor doses and lactate levels were also faster in ECMO survivors.
Outcomes and complications
Patients treated with or without ECMO had the same hospital mortality rate (56%, 5/9, Table 1). The five ECMO non-survivors died during therapy after a median (range) of 5 (2–25) days, two of intractable hypoxaemia and three of multiorgan failure. The four ECMO survivors had median (range) duration of ECMO therapy of 9 (4–14) days and were ventilated for median (IQR) duration of 25 (22–33) days after stopping ECMO. Age of ECMO survivors was similar to non-survivors [42 (25–57) versus 49 (36–51) years], as was BMI [27 (23–30) versus 30 (29–42) kg/m2]. However, a lower peak lactate level [2 (1.7–3.5) versus 12.6 (4.9–13.8) mmol/l, p < 0.05] and a non-statistically significant trend to a lower SAPS II score at ICU admission [34 (20–47) versus 53 (47–59)] were observed in survivors. Despite this, ECMO survivors did not present less severe respiratory failure before ECMO (Table 3) or lower Lung Injury Score [3.4 (3.2–3.6) versus 3.6 (3.5–3.7)]. Of the three patients receiving veno-arterial ECMO, two survived compared with only two of the six patients receiving veno-venous ECMO. Among the five non-survivors treated without ECMO, four died of multiorgan failure and one of cardiac arrest due to hypoxaemia before ECMO could be performed, after median (IQR) duration of mechanical ventilation of 8 (2–8) days.
Haemorrhagic complications occurred in four patients under ECMO: respiratory tract haemorrhage in three, epistaxis requiring nasal packing in one, and diffuse bleeding due to fibrinolysis, including haemothorax, occurring 10 days after surgical lung biopsy in one. This latter complication occurred after 13 days of ECMO in the post partum patient concomitant with ECMO weaning; it was followed by a favourable outcome without surgical treatment. While under ECMO, patients were transfused a median (IQR) of 7 (4–15) units of red blood cells (1 (1–1) U/day), 3 (2–4) units of frozen plasma and 1 (0–1) unit of platelets. Patients with respiratory tract haemorrhage or epistaxis required daily, but not massive, red blood cell transfusion. The patient presenting with fibrinolysis received six red blood cell, six frozen plasma and one platelet unit(s). In one case, membrane exchange was required during ECMO therapy.
Discussion
The results of this observational study show that the implementation of an ECMO-based management protocol including a mobile unit allowing ECMO initiation in referral hospitals followed by transfer to a reference centre resulted in an acceptable mortality rate in patients with influenza (H1N1)-related intractable respiratory failure.
ECMO is expected to be of greatest benefit when initiated early in the course of severe lung injury. Indeed, several reports have clearly shown that survival rate decreases as a function of the period of mechanical ventilation before institution of ECMO [4, 15, 16]. A possible reason for this is the impossibility of applying protective ventilation without ECMO in the most severe cases, which could result in ventilator-associated lung injury and in the initiation of multisystem disease. In the present study, patients treated with ECMO had not only refractory respiratory failure but also higher severity scores than patients not requiring ECMO, due to the severity of pneumonia. Moreover, ECMO non-survivors required much higher doses of norepinephrin and had higher lactate levels before ECMO initiation. This latter result confirms that ECMO may be less useful in patients with very severe shock and where multiorgan failure is already initiated. Although early ECMO was possible in the most severe cases through rapid intervention of our mobile team, it must be remembered that it was performed as a salvage therapy in at least two patients for whom multisystem disease had already initiated. The advantage of a mobile team allowing patient transfer under ECMO to a reference centre has previously been suggested [4]. Its potential benefit may be reinforced for fulminating ARDS such as that encountered with influenza H1N1. In the present study, it allowed ECMO initiation in referral hospitals for six patients who could not have been transported otherwise.
Although the severity of hypoxaemia or respiratory acidosis before ECMO was not different between ECMO survivors and non-survivors, we observed a greater improvement in PaO2 in survivors rapidly after ECMO initiation. Severity of hypoxaemia after ECMO initiation has previously been suggested to be linked with prognosis. Indeed, in a recent multicentre database report [16], FiO2 required after 24 h of ECMO was higher in ECMO non-survivors.
In a multicentre observational study, 68 patients were reported as treated with ECMO for influenza H1N1-induced ARDS in Australian and New Zealand ICUs [8]. The mortality rate reported was 21%, although a few patients were still in ICU. Although our study was a single-centre study of only nine patients treated with ECMO, several other reasons could explain the discrepancy between the 56% mortality rate that we report and the much lower rate reported in Australia and New Zealand. First, we used inclusion criteria for ECMO based on a recent multicentre randomized controlled study [5]. In this study, a 37% ICU mortality rate was reported for 90 ARDS patients treated with ECMO. Conversely, no inclusion protocol was reported in the Australia/New Zealand report on ECMO for influenza H1N1 ARDS [8]. This could have resulted in application of ECMO therapy in less severely affected patients. There is evidence of this: median (IQR) PaCO2 was 69 (54–86) mmHg in the Australian report [4], whereas it was 85 (69–91) mmHg in our patients. PaCO2 >70 mmHg has been associated with increased mortality in patients treated with ECMO [16]. Moreover, although patients with SOFA score >16 were not considered for ECMO therapy, decisions to use ECMO were always taken with extreme urgency in patients who had just been mechanically ventilated. In these conditions, early applied severity scores may not reflect exactly the risk of evolution to multiorgan failure. As a result, multiorgan failure contributed to death in most of our ECMO non-survivors, whereas no patients died of multiorgan failure in the Australia/New Zealand cohort [8]. Additionally, only five patients treated with ECMO (8%) required renal replacement therapy in the latter study, whereas it was necessary in five (56%) of our patients. Finally, our centre is considered as a regional reference centre for treatment of the most severe cases, which could explain the high levels of severity seen in our patients. We therefore cannot exclude that less severely ill patients might benefit from ECMO with a better outcome. Nonetheless, like us, most physicians will be concerned by the high mortality rate reported here in patients treated both with and without ECMO. Treatment decisions were always taken by a multidisciplinary team comprising intensivists and cardiac surgeons, ruling out systematic errors in support. Finally, due to the low number of patients in our cohort, mortality comparisons with larger populations of ARDS patients are difficult. Nevertheless, the relatively high proportion of patients with severe comorbidities such as cancer probably contributed to our high mortality rate, particularly in the group of patients not receiving ECMO.
We used wire-reinforced 20 Fr cannulae to prevent kinking. The thin-wall technology allows higher flow rates with smaller cannulae. These allow a single cannula to be used while maintaining sufficient flow. Their use may help prevent venous injury and thrombosis. However, whether their use induces very strong suction pressures which may induce coagulation disorders remains to be evaluated.
Corticosteroids were notably administered in patients with persistent ARDS, preventing ECMO weaning after 7–10 days. In one patient who survived, they were given based on the result of a surgical lung biopsy showing recent lung fibrosis. The four other patients who received steroids under ECMO died. Post mortem biopsy performed in two of them revealed lung fibrosis.
In conclusion, ECMO may be an effective salvage treatment for patients with influenza A (H1N1)-related ARDS presenting rapid refractory respiratory failure, particularly when provided early in a specialized reference centre.
References
Zapol WM, Snider MT, Hill JD, Fallat RJ, Bartlett RH, Edmunds LH, Morris AH, Peirce EC 2nd, Thomas AN, Proctor HJ, Drinker PA, Pratt PC, Bagniewski A, Miller RG Jr (1979) Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 242:2193–2196
Morris AH, Wallace CJ, Menlove RL, Clemmer TP, Orme JF Jr, Weaver LK, Dean NC, Thomas F, East TD, Pace NL, Suchyta MR, Beck E, Bombino M, Sittig DF, Böhm S, Hoffmann B, Becks H, Butler S, Pearl J, Rasmusson B (1994) Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 149:295–305
Lindén V, Palmér K, Reinhard J, Westman R, Ehrén H, Granholm T, Frenckner B (2000) High Survival in adult patients with acute respiratory distress syndrome treated by extracorporeal membrane oxygenation, minimal sedation, and pressure supported ventilation. Intensive Care Med 26:1630–1637
Hemmila MR, Rowe SA, Boules TN, Miskulin J, McGillicuddy JW, Schuerer DJ, Haft JW, Swaniker F, Arbabi S, Hirschl RB, Bartlett RH (2004) Extracorporeal life support for severe acute respiratory distress syndrome in adults. Ann Surg 240:595–607
Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D, CESAR trial collaboration (2009) Efficacy and economic assessment of conventional ventilatory support versus Extracorporeal Membrane Oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 374:1351–1363
World Health Organization. Swine flu illness in the United States and Mexico-update 2. Available via DIALOG http://www.who.int/csr/don/2009_04_26/en/index.html Accessed 26 April 2009
Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quiñones-Falconi F, Bautista E, Ramirez-Venegas A, Rojas-Serrano J, Ormsby CE, Corrales A, Higuera A, Mondragon E, Cordova-Villalobos JA, INER Working Group on Influenza (2009) INER Working Group on Influenza. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med 361:680–689
The Australia New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza investigators (2009) Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 302:1888–1895
Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, Stelfox T, Bagshaw S, Choong K, Lamontagne F, Turgeon AF, Lapinsky S, Ahern SP, Smith O, Siddiqui F, Jouvet P, Khwaja K, McIntyre L, Menon K, Hutchison J, Hornstein D, Joffe A, Lauzier F, Singh J, Karachi T, Wiebe K, Olafson K, Ramsey C, Sharma S, Dodek P, Meade M, Hall R, Fowler RA, Canadian Critical Care Trials Group H1N1 Collaborative (2009) Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 302:1872–1879
Institut de Veille Sanitaire. Bulletin épidémiologique grippe A (H1N1) au 21 avril 2010. Available via DIALOG http://www.invs.sante.fr/display/?doc=surveillance/grippe_dossier/ Accessed 21 April 2010
Freed DH, Henzler D, White CW, Fowler R, Zarychanski R, Hutchison J, Arora RC, Manji RA, Legare JF, Drews T, Veroukis S, Kesselman M, Guerguerian AM, Kumar A; Canadian Critical Care Trials Group (2010) Extracorporeal lung support for patients who had severe respiratory failure secondary to influenza A (H1N1) 2009 infection in Canada. Can J Anaesth 57:240–247
Murray JF, Matthay MA, Luce JM, Flick MR (1988) An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 138:720–723
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG, On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med 22:707–710
Pranikoff T, Hirschl RB, Steimle CN, Anderson HL 3rd, Bartlett RH (1997) Mortality is directly related to the duration of mechanical ventilation before the initiation of extracorporeal life support for severe respiratory failure. Crit Care Med 25:28–32
Brogan TV, Thiagarajan RR, Rycus PT, Bartlett RH, Bratton SL (2009) Extracorporeal membrane oxygenation in adults with severe respiratory failure: a multi-centre database. Intensive Care Med 35:2105–2114
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roch, A., Lepaul-Ercole, R., Grisoli, D. et al. Extracorporeal membrane oxygenation for severe influenza A (H1N1) acute respiratory distress syndrome: a prospective observational comparative study. Intensive Care Med 36, 1899–1905 (2010). https://doi.org/10.1007/s00134-010-2021-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-2021-3