Abstract
Purpose
Bohr’s dead space (VDBohr) is commonly calculated using end-tidal CO2 instead of the true alveolar partial pressure of CO2 (PACO2). The aim of this work was to validate VDBohr using PACO2 derived from volumetric capnography (VC) against VDBohr with PACO2 values obtained from the standard alveolar air formula.
Methods
Expired gases of seven lung-lavaged pigs were analyzed at different lung conditions using main-stream VC and multiple inert gas elimination technique (MIGET). PACO2 was determined by VC as the midpoint of the slope of phase III of the capnogram, while mean expired partial pressure of CO2 (PeCO2) was calculated as the mean expired fraction of CO2 times the barometric minus the water vapor pressure. MIGET estimated expired CO2 output (VCO2) and PeCO2 by its V/Q algorithms. Then, PACO2 was obtained applying the alveolar air formula (PACO2 = VCO2/alveolar ventilation).
Results
We found close linear correlations between the two methods for calculating both PACO2 (r = 0.99) and VDBohr (r = 0.96), respectively (both p < 0.0001). Mean PACO2 from VC was very similar to the one obtained by MIGET with a mean bias of −0.10 mmHg and limits of agreement between −2.18 and 1.98 mmHg. Mean VDBohr from VC was close to the value obtained by MIGET with a mean bias of 0.010 ml and limits of agreement between −0.044 and 0.064 ml.
Conclusions
VDBohr can be calculated with accuracy using volumetric capnography.
Similar content being viewed by others
Introduction
For calculating true dead space, Bohr’s formula uses alveolar (PACO2) and mixed expired partial pressures of CO2 (PeCO2) as:
[1] assuming that CO2 in the inspired gases is zero. The measurement of PACO2 is technically difficult and has caused hot debates in the past. Haldane and Priestley [2] believed that representative alveolar CO2 samples could only be obtained at the end of a forced expiration. Later, Krogh and Lindhard [3] showed that alveolar CO2 reaches a plateau by the end of expiration once the volume of gas in the anatomical dead space has been washed out. Fowler [4] confirmed the latter concept by his famous analysis, which to date is considered the reference method for separating alveolar gases from those in the main airways.
Aitken and Clark-Kennedy[5] were the first to suggest that mean PACO2 can be obtained using volumetric capnography (VC). Fletcher et al. [6] confirmed this hypothesis on theoretical grounds describing that mean PACO2 could be measured directly as the midpoint of phase III of VC, a concept that Breen et al. [7] have been supporting over the the last years. To our knowledge, this proposed measurement method for PACO2 has, however, not yet been duly validated. This could be the reason why PACO2 is still erroneously being replaced by PaCO2 or PETCO2 in Bohr’s formula. Therefore, the aim of the study was to compare Bohr’s dead space using PACO2 values from capnograms with the one in which PACO2 was derived from the standard alveolar air formula:
where VCO2 is the amount of CO2 eliminated by the lungs and VA is the alveolar ventilation. VCO2 was calculated independently applying the mathematical algorithm of the multiple inert gas elimination technique (MIGET) [10, 11].
Methods
We re-analyzed data of seven lung-lavaged pigs in which MIGET was performed, and other data than those presented here have been submitted for publication elsewhere. Anesthetized animals were ventilated using a volume-control mode with fixed settings of tidal volume of 6 ml/kg, 30 breaths/minute, I:E of 1:2 and FiO2 of 1. Positive end- expiratory pressures (PEEP) from 10 to 22 cmH2O were randomly assigned to intentionally induce different degrees of lung aeration and V/Q ratios.
Standard ECG, transcutaneous O2 saturation by pulse oximetry and invasive blood pressure via a femoral catheter were monitored. Volumetric capnography (the dynamic measurement of expired CO2 vs. expired volume; called VC in the following text) was recorded by a NICO capnograph (Respironics, Wallingford, CT). CO2 was measured by an infrared mainstream sensor Capnostat 3 (response time <60 ms and resolution 2 mmHg) placed at the airway opening.
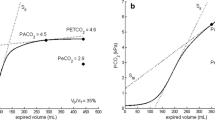
Volumetric capnography was fitted by Levenberg-Marquardt curves using a custom-made MatLab program (Mathworks, Natick, MA) [8], and parameters were mathematically derived from them (for details, see on-line suplement). Briefly, the first step was to determine the limit between airway dead space and alveolar tidal volume, which according to Fowler’s [4] concept is represented by the midpoint of phase II. This midpoint of phase II is the inflection point of the whole capnogram where its curvature changes sign. Our algorithm determines this inflection point as the maximum of the first derivative of the mathematical function fitted to the set of data points comprising the capnogram of each breath. Then, PACO2 was determined from VC as the midpoint of the line of the slope of phase III, starting at the inflection point of the capnogram and terminating at PETCO2.
Alveolar tidal volume multiplied by the respiratory rate determines alveolar ventilation (VA). The elimination of CO2 (VCO2) was calculated as the area under the curve of VC multiplied by the respiratory rate. PETCO2 is the partial pressure of CO2 found at the end of expiration. Mean PeCO2 was determined by multiplying the fractional expired CO2 concentration by the barometric pressure minus the water vapor pressure [9].
VCO2 and PeCO2 were also calculated independently from MIGET’s V/Q analysis in the way described by West [10] and applied by Wagner et al. [11]. We used the VCO2 to calculate PACO2 by the alveolar gas formula.
Expired gases for MIGET analysis were sampled via a heated mixing box connected to the ventilator’s exhaust valve. Arterial blood samples were obtained simultaneously and analyzed immediately by an ABL 300 (Radiometer, Copenhagen, Denmark). Thirty capnograms belonging to this sampling period were analyzed, and a representative mean cycle was calculated.
Pearson’s linear correlation coefficients were calculated. The degree of agreement between the two techniques was calculated as outlined by Bland-Altman.
Results
A total of 49 sets of measurements were analyzed. The table shows the variables studied and the value of VDBohr calculated from the PACO2 and PeCO2 values obtained by the two methods (Table 1).
We found close correlations between the parameters PACO2 and VDBohr (Fig. 1) and between PeCO2 and VCO2 (Fig. 2 on-line supplement) measured by VC and mathematically derived from MIGET.
Mean PACO2 from VC was very similar to the value obtained by MIGET with a mean bias of −0.10 mmHg and limits of agreement between −2.18 and 1.98 mmHg. PeCO2 for VC and MIGET showed a mean bias of −0.49 mmHg and limits of agreement between −2.45 and 1.47 mmHg. The VCO2 measured by VC was higher than the corresponding value calculated by MIGET with a mean bias of −4.2 mL/min and limits of agreement between −19.2 and 10.8 ml/min. Mean VDBohr from VC was very similar to the value obtained by MIGET with a mean bias of 0.010 ml and limits of agreement between −0.044 and 0.064 ml.
Discussion
These results show that VDBohr can be measured directly and with accuracy using VC. The mean PACO2 found at the midpoint of the slope of the phase III of the capnograms was well correlated with the one obtained by the alveolar air equation using VCO2 values provided by MIGET.
The normal variations in the alveolar CO2 tension during the respiratory cycle (decrease in inspiration and increase in expiration) make it difficult to obtain physical gas samples truly representative of alveolar CO2. Therefore, mean or average PACO2 is the value to be used in any physiological calculations where alveolar air comes into play, hoping that this will make dead space calculations more reliable and reproducible. Since capnographs placed at the airway opening usually do not sample CO2 values during inspiration, the measurement of mean PACO2 is performed during the expiratory time only. Already as early as 1951, Dubois et al. [12] pointed out that mean PACO2 is best represented by an alveolar sample taken shortly after mid-expiratory time.
Aitken and Clark-Kennedy [5] originally described a method to obtain alveolar gas using volumetric capnograms during excercise. Fletcher et al. [6] took this principle a step further and suggested the calculus of alveolar dead space using the mean value of PACO2 measured directly from VC. They reasoned that the mean PACO2 is represented by the midpoint of the slope of phase III of the alveolar compartment. This idea was applied by Breen et al. [7] using the PAECO2, another acronym for mean PACO2. Breen et al. [7] highlighted the differences between the PACO2 measured by time-based and by volume-based capnography pointing out that only if CO2 were weighted by the exhaled volume, PACO2 could be found in the midpoint of phase III. For this reason, methods estimating PACO2 on a time basis are not accurate enough to accomplish this task [13].
To our knowledge much of the above reasoning is based on theoretical concepts, assumptions and measurements using old technologies, but has never been adequately validated. Therefore, we used the alveolar air formula as reference, which is based on the mathematical principle that PACO2 depends on the ratio between the amount of CO2 eliminated by the lungs and the alveolar ventilation.
Our results support the following concepts of VDBohr:
First, Enghoff’s modification of Bohr’s formula using the concept of ideal PACO2 (PACO2 = PaCO2) described by Riley is still an approximation because, even in healthy lungs, anatomical dead space is always present, and anatomical shunt increases the difference between PaCO2 and PACO2 [14, 15]. Therefore, the Bohr-Enghoff formula includes the effect of any venous admixture within the lungs, giving a higher value than VDBohr. This overestimated dead space has been called shunt dead space by Suter et al. [16], although it has little to do with the classical concept of dead space in the sense of a ventilated but not perfused lung unit [17].
Our experimental data support the above statement. At all times PaCO2 was higher than PACO2 because of the V/Q mismatch induced by the different degrees of lung overdistension and collapse. Thus, in real lungs the value of PaCO2 and PACO2 cannot be assumed to be equal.
Second, an error in VDBohr is also observed when in Bohr’s formula PETCO2 is used instead of PACO2 as the slope of phase III is positive in almost all patients. PETCO2 will overstimate the real VDBohr value because PETCO2 > PACO2. For example, our data show that VDBohr based on PETCO2 was 0.61 ± 0.05, a value that was 9 and 13% higher than VDBohr obtained using PACO2 from VC and from MIGET, respectively.
Third, considering the previous point, the popular index Pa-ETCO2 should become more reliable and physiologically more correct if it were re-written as Pa-ACO2 since both PaCO2 and PACO2 are representing mean CO2 values in the blood and the alveolar compartment, respectively. PETCO2, on the other hand, represents only a group of alveoli with low V/Q ratios and/or low expiratory time constants, which contribute high concentrations of CO2 at the end of expiration.
Fourth, our data confirm the results of previous validations of VC-derived PeCO2, such as the one published by Kallet et al. [9].
We conclude that the original Bohr formula can be applied on a breath-by-breath basis and with accuracy if PACO2 is determined from the midpoint of the slope of phase III of the volumetric capnogram. PACO2 should neither be replaced by PETCO2 nor by the invasive PaCO2. The proposed measurements of PACO2 are highly dependent on a robust determination of the slope of phase III. As long as such breath-by-breath features are not available for bedside use, clinicians are left with the manual calculation of PACO2. However, using the alveolar air formula with VCO2 and VA coming from volumetric capnography, determination of dead space becomes easy and entirely non-invasive.
References
Bohr C (1891) Über die Lungeatmung. Skand Arch Physiol 2:236–238
Haldane JS, Priestley JG (1905) The regulation of the lung-ventilation. J Physiol 32:225–266
Krogh A, Lindhard J (1913) On the average composition of the alveolar air and its variations during the respiratory cycle. J Physiol 47:431–445
Fowler WS (1948) Lung function studies II. The respiratory dead space. Am J Physiol 154:405–416
Aitken RS, Clark-Kennedy AE (1928) On the fluctuation in the composition of the alveolar air during the respiratory cycle in muscular excercise. J Physiol 65:389–411
Fletcher R, Jonson B (1981) The concept of deadspace with special reference to the single breath test for carbon dioxide. Br J Anaesth 53:77–88
Breen PH, Mazumdar B, Skinner SC (1996) Comparison of end-tidal PCO2 and average alveolar expired PCO2 during positive end-expiratory pressure. Anesth Analg 82:368–373
Tusman G, Scandurra A, Böhm SH, Suarez Sipmann F, Clara F (2009) Model fitting of volumetric capnograms improves calculations of airway dead space and slope of phase III. J Clin Monit Comput 23:197–206
Kallet RH, Daniel BM, Garcia O, Matthay MA (2005) Accuracy of physiologic dead space measurements in patients with ARDS using volumetric capnography: comparison with the metabolic monitor method. Respir Care 50:462–467
West JB (1969) Ventilation-perfusion inequality and overall gas exchange in computer models of the lung. Respiration Physiol 7:88–110
Wagner PD, Saltzman HA, West JB (1974) Measurement of continuous distributions of ventilation-perfusion ratios: theory. J Appl Physiol 36:588–599
Dubois AB, Britt AG, Fenn WO (1951) Alveolar CO2 during the respiratory cycle. J Appl Physiol 4:535–548
Jordanoglou J, Koulouris N, Kyroussis D, Rapakoulias P, Vassalos P, Madianos J (1995) Measurement of effective alveolar carbon dioxide tension during spontaneous breathing in normal subjects and patients with chronic airways obstruction. Thorax 50:240–244
Enghoff H (1938) Volumen inefficax. Bemerkungen zur Frage des schädlichen Raumes. Uppsala Läkareforen Forhandl 44:191–218
Riley RL, Cournand A (1949) Ideal alveolar air and the analysis of ventilation-perfusion relationships in the lungs. J Applied Physiol 1:825–847
Suter PM, Fairley HB, Isenberg MD (1975) Optimum end-expiratory airway pressure in patients with acute pulmonary failure. N Engl J Med 292:284–289
Hedenstierna G, Sandhagen B (2006) Assessing dead space. A meaningful variable? Minerva Anestesiol 72:521–528
Acknowledgments
We thank Dr. Peter Wagner for providing us with invaluable insights into the MIGET calculations and Adriana Scandurra and Gustavo Meschino for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tusman, G., Sipmann, F.S., Borges, J.B. et al. Validation of Bohr dead space measured by volumetric capnography. Intensive Care Med 37, 870–874 (2011). https://doi.org/10.1007/s00134-011-2164-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2164-x