Abstract
Purpose
Since 1997, we have routinely used prone positioning (PP) in patients who have a PaO2/FiO2 below 100 mmHg after 24–48 h of mechanical ventilation and who are ventilated using a low stretch ventilation strategy. We report here the characteristics and prognosis of this subgroup of patients with severe lung injury to illustrate the feasibility, role, and impact of routine PP in acute respiratory distress syndrome (ARDS).
Results
A total of 218 patients were admitted because of ARDS between 1997 and 2009. Of these patients, 57 (26%) were positioned prone because of a PaO2/FiO2 below 100 mmHg after 24–48 h of mechanical ventilation. Age was 51 ± 16 years, PaO2/FiO2 74 ± 19, and PaCO2 54 ± 10 mmHg. The lung injury score was 3.13 ± 0.15. Tidal volume was 7 ± 2 mL/kg, PEEP 5.6 ± 1.2 cmH2O, and plateau pressure 27 ± 3 cmH2O. Prone sessions lasted 18 h/day and 3.4 ± 1.1 sessions were required to obtain an FiO2 below 60%. The 60-day mortality was 19% and death occurred after 12 ± 5 days. The ratio between observed and predicted mortality was 0.43. In patients with a PaO2/FiO2 below 60 mmHg, the 60-day mortality was 28%. Logistic regression analysis showed that among the 218 patients, PP appeared to be protective with an odds ratio of 0.35 [0.16–0.79].
Conclusion
We demonstrate the clinical feasibility of routine PP in patients with a PaO2/FiO2 below 100 mmHg after 24–48 h and suggest that, when combined with a low stretch ventilation strategy, it is protective with a high survival rate.
Similar content being viewed by others
Introduction
Despite the use of protective ventilation, mortality remains elevated in the most severe form of acute respiratory distress syndrome (ARDS) [1]. This encourages some to propose venoarterial extracorporeal membrane oxygenation (ECMO) in this situation [2–4]. However, prone positioning (PP) could be an alternative in the management of patients with severe ARDS. Despite a recent meta-analysis which demonstrated its ability to improve prognosis in patients with a PaO2/FiO2 below 100 mmHg [5], PP is often considered as a “rescue” therapy and is not used in many intensive care units (ICUs). In the Australian–New Zealand study performed during the 2009 influenza (H1N1) epidemic, only 20% of patients underwent PP before ECMO [4].
Since 1997, we have routinely used PP in hemodynamically stable patients with a PaO2/FiO2 below 100 mmHg after 24–48 h of mechanical ventilation. We report here the characteristics and prognosis of this subgroup of patients with severe lung injury to illustrate the feasibility, role, and impact on prognosis of routine PP in ARDS.
Materials and methods
Patients
Since 1997, in patients admitted to our ICU because of ARDS, we have prospectively recorded epidemiological characteristics, simplified acute physiologic score (SAPS II) [6], lung injury score (LIS) [7], blood gas analysis, plateau pressure, and respiratory settings. At the same time, these cases have all been entered with a main diagnosis of ARDS in the database of the CUB-Réa Network, which was set up by intensivists of hospitals in Paris and its suburbs [8]. ARDS was defined using the criteria proposed by the American–European consensus conference [9]. All patients had an acute onset of respiratory failure, bilateral chest infiltrates, a PaO2/FiO2 below 200 mmHg during mechanical ventilation regardless of PEEP, and no evidence of increased pulmonary venous pressure. In particular, we report here patients who underwent PP, i.e., the patients with the most severe ARDS, with a PaO2/FiO2 which remained below 100 mmHg after 24–48 h of mechanical ventilation. The standard mortality ratio (SMR) of this population was obtained by dividing the observed mortality by the mortality predicted by the SAPS II.
Clinical management
All patients were managed as follows:
-
Mechanical ventilation was performed by applying a low stretch ventilation strategy, as has been done in our ICU since 1993 [10]. Briefly, this combined a tidal volume of 6–9 mL/kg of measured body weight, an inspiratory/expiratory ratio of 1:2, an end-inspiratory pause of 0.5 s, and a respiratory rate adapted to the PaCO2 level without inducing or increasing any intrinsic PEEP per se. PEEP was selected to produce better oxygenation without worsening hemodynamics, and to match intrinsic PEEP when present [11]. In the case of a PaCO2 above 50–55 mmHg, the heat and moisture exchanger was removed and patients were ventilated using a heated humidifier [12].
-
PP was routinely used in patients with a PaO2/FiO2 below 100 mmHg after 24–48 h of mechanical ventilation, provided that hemodynamics were stabilized. Hemodynamic stability was defined as a systolic blood pressure above 90 mmHg, whatever the need for drugs. During PP, we never used thoracopelvic support. Each prone position session lasted about 18 h/day, from about 3 p.m. to 8 a.m. the day after. Prone position was continued until patients were ventilated with an FiO2 below 60%. Severe adverse effects of PP, e.g., accidental extubation, was systematically recorded.
-
Hemodynamic optimization was achieved using a radial artery catheter and at least one transesophageal echocardiography per day during the first 3 days.
-
Finally, renal replacement therapy was applied if necessary using continuous veno-venous hemodiafiltration.
Statistical analysis
Statistical analysis was performed in collaboration with the Ambroise Paré Hospital Department of Biostatistics, using the Statview 5.0 (SAS Institute, Cary, NC, USA). Values, when normally distributed, are presented as mean ± standard deviation. One-way analysis of variance (ANOVA) was used to compare PaO2/FiO2, PaCO2, plateau pressure, and compliance of the respiratory system. We also looked for an interaction between the year of inclusion and mortality to assess the potential effect of the learning curve. Finally, we compared data between survivors and nonsurvivors through use of the chi-squared test with Yates’ correction or Fisher’s exact test when necessary for categorical variables, and with a t test for continuous variables. We entered four variables found to be significantly associated with death into a logistic regression model. A two-tailed p value of less than 0.05 was considered statistically significant.
Results
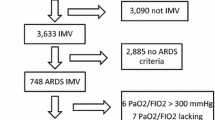
From January 1997 to December 2009, 218 patients admitted to our unit were entered in the database of the CUB-Réa Network with a main diagnosis of ARDS (Fig. 1). Mean age was 57 ± 16 years and 62% of patients were male. SAPS II was 58 ± 22. Eighty-four percent of patients required infusion of vasopressor and 44% renal replacement therapy. Mortality rate at day 60 was 38.5%, leading to an SMR of 0.60 (Fig. 1).
Of these patients, 57 (26%) had a PaO2/FiO2 below 100 mmHg after 24–48 h of ventilation and were then systematically positioned prone (Fig. 1), 23 during the 1997–2002 period and 17 during the 2003–2006 and 2007–2009 periods. Age was 51 ± 16 years. Thirty patients (53%) were male. SAPS II, calculated during the first 24 h following admission, was 49 ± 14. Most patients (91%) had ARDS of pulmonary origin, mainly due to bacterial pneumonia (n = 32) and aspiration (n = 13). At admission mean PaO2/FiO2 was 100 ± 53 mmHg, PaCO2 50 ± 11 mmHg and pH 7.32 ± 0.11. Fifty-eight percent of patients required infusion of epinephrine or norepinephrine and 42% required veno-venous hemodiafiltration.
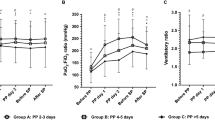
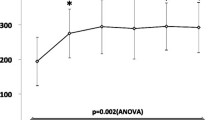
Characteristics of patients and respiratory settings on the day of PP were as follows. Tidal volume was 473 ± 134 mL (7 ± 2 mL/kg), respiratory rate 18 ± 4 per min, and PEEP 5.6 ± 1.2 cmH2O. PaO2/FiO2 and PaCO2 were respectively 74 ± 19 and 54 ± 10 mmHg and pH was 7.26 ± 0.09. Plateau pressure was 27 ± 3 cmH2O for a compliance of the respiratory system of 21 ± 8 mL/cmH2O. LIS was 3.13 ± 0.15. Fourteen patients (25%) had a PaO2/FiO2 below 60 mmHg, 25 (44%) a PaO2/FiO2 between 60 and 80 mmHg, and 18 (31%) a PaO2/FiO2 above 80 mmHg. To achieve an FiO2 below 60%, 3.4 ± 1.1 PP sessions were needed. One patient required 1 session, 8 patients 2 sessions, 30 patients 3 sessions, 9 patients 4 sessions, 6 patients 5 sessions, and finally 3 patients 6 sessions; thus, 84% of patients had 3 or more sessions. Neither accidental extubation nor severe hemodynamic compromise was observed when turning the patients prone. Figure 2 reports PaO2/FiO2, PaCO2, plateau pressure, and compliance of the respiratory system at day 1, on the day of PP, and at the end of the prone position sessions.
Box and whisker plots of PaO2/FiO2, PaCO2, plateau pressure, and compliance of the respiratory system (Crs) at day 1 (D1), on the day of prone positioning (DPP), and at the end of prone ventilation sessions (DEND). Horizontal line inside the box = median. *p < 0.05 using one-way analysis of variance (ANOVA)
The duration of mechanical ventilation was 24 ± 19 days. Eleven patients died after 12 ± 5 days, leading to a 19% mortality rate at day 60, for a predicted mortality rate of 44%. The SMR was 0.43. No interaction was found between the year of inclusion and the mortality rate (p = 0.148). Mortality rate was 28% in patients who had a PaO2/FiO2 below 60 mmHg, 16% in patients who had a PaO2/FiO2 between 60 and 80 mmHg, and 17% in patients who had a PaO2/FiO2 above 80 mmHg. Table 1 reports the main characteristics and causes of death in these patients. Among the overall population of 218 ARDS patients, age and the severity of circulatory failure were associated with death at day 60 (Table 2). Prone position appeared to be protective (odds ratio 0.35, p = 0.01, Table 2).
Discussion
We report here our 13-year experience of ARDS management combining a low stretch ventilation strategy with ventilation in the prone position in patients with the most severe ARDS. This strategy resulted in 26% of our ARDS patients being positioned prone. Mortality rate was 19% in patients with a PaO2/FiO2 below 100 mmHg and 28% in those with a PaO2/FiO2 below 60 mmHg. The SMR was 0.43 and PP appeared to be protective in a multivariate logistic regression analysis.
PP in ARDS was first proposed in 1977 [13]. However, randomized controlled studies in adults have failed to demonstrate improvement in prognosis [14–16]. Many explanations have been advanced: a lack of power in the Spanish study [16], too short a PP session, i.e., 6 h, in the Italian study [15], and too heterogeneous a population in the French study [14]. In fact, the Italian study suggested a gain in the subgroup of patients with a PaO2/FiO2 below 100 mmHg [15]. Very recently, in a meta-analysis including 10 studies, Sud et al. [5] demonstrated that prone ventilation reduces the relative risk of death in patients with severe hypoxemia, i.e., those with a PaO2/FiO2 below 100 mmHg, by 16%. However, PP is often considered as a rescue therapy and is not used in many ICUs. Our results suggest that such a strategy is useful, may save lives, and could be included in the routine therapeutic protocol of ICUs. We perform the procedure without thoracopelvic support, with prone position sessions of 18 h/day. Thoracopelvic support has been reported to be poorly tolerated hemodynamically [17]; and in their meta-analysis, Sud et al. [5] reported that prone position lasting longer than 14 h tends to have an impact on mortality.
In the recent CESAR study, which compared ECMO with conventional strategy in ARDS, inclusion criteria were age 18–65 years and LIS greater than 3.0, or greater than 2.5 if the patients continued to deteriorate [3]. Severity was evaluated after about 35 h of mechanical ventilation, in hemodynamically stable patients, as in our strategy. The mean PaO2/FiO2 was 76 mmHg, very close to ours, and the mortality rate at 6 months was 37% in the ECMO group and 45% in the control group. Interestingly, the response to prone position was not taken into account to assess the need for ECMO and few patients actually had ventilation in the prone position [3]. In the recent Australian–New Zealand experience in flu (H1N1) ARDS, 61 patients underwent ECMO [4]. The lowest median PaO2/FiO2 was 56 mmHg and LIS was 3.8 with an average PEEP of 18 cmH2O (3.13 in our study with an average PEEP of 5.6 cmH2O). Only 20% of the patients were ventilated in the prone position before deciding to start ECMO [4]. Finally, Roch et al. [18] recently reported a prospective observational comparative study in 18 cases of severe influenza A (H1N1) ARDS. In the ECMO group, in-hospital mortality was 56% in patients with the same SAPS II but a higher LIS [18]. Interestingly, in the non-ECMO group, with less critically ill patients than in our series, in-hospital mortality was also 56% and nothing was said regarding the use of PP [18]. However, the H1N1 population is probably different from other types of ARDS [4], rendering any definitive comparison between these studies and our results difficult.
The low mortality rate of our study can be directly attributed to prone ventilation, as suggested by our logistic regression of factors of mortality, but also to our associated low stretch ventilation strategy, with a plateau pressure below 28 cmH2O, a low PEEP (5–7 cmH2O), and controlled hypercapnia [10]. Prone ventilation allowed us to improve oxygenation and to recruit the lung without increasing PEEP and plateau pressure [19]. In a previous study, Villar et al. [20] reported mortality as high as 68% in patients with a PaO2/FiO2 below 150 mmHg after 24 h of a nonprotective mechanical ventilation. Interestingly, in the most recent trial conducted in patients with severe hypoxemia, prone ventilation did not provide any benefit, despite its use within 72 h of diagnosis and a session lasting up to 20 h/day [21]. The 28-day mortality rate was 37.8%. However, oxygenation improvement due to PP did not lead to a decrease in PEEP, which remained close to 14 cmH2O [21]. This may contribute to persistent high lung stress, counterbalancing the beneficial effects of prone ventilation.
Despite our low mortality rate, 11 of the 57 patients died. This raises the question of the place of ECMO in these cases. Interestingly, our patients died 12 ± 5 days after admission, most from a complication acquired in the ICU and not from refractory hypoxemia, suggesting the futility of such a procedure in this subgroup. However, our study is clearly not large enough to answer to this question.
One can say that the relatively low PEEP used in our patients may overestimate the level of lung injury when compared with other series using a PEEP above 10 cmH2O. The response to standard ventilator settings, 24 h after meeting ARDS criteria, has been proposed by Villar et al. [1] to separate patients according to the degree of lung damage. Patients in whom a PEEP of 10 cmH2O or above with an FiO2 of 0.5 or more increased the PaO2/FiO2 above 200 mmHg had a significantly lower mortality compared with the others. In the latter group of established ARDS, in-hospital mortality was 45.5% [1]. Interestingly, our patients were also selected after 24–48 h of ventilation. However, despite a median PEEP of 5.6 cmH2O, the mean LIS was 3.13. In the study by Villar et al. [1], the mean LIS in patients with established ARDS was 2.9. Estenssoro et al. [22] also reported how different levels of PEEP may change the evaluation of lung injury based on the PaO2/FiO2 ratio. But, they demonstrated that LIS remains stable whatever the PEEP [22].
The main limitation of our study is its observational and uncontrolled design, without a control group. As shown in the flow chart of our study, the mortality rate of patients with ARDS who were not positioned prone was higher, but most of these patients had severe hemodynamic compromise with a PaO2/FiO2 above 100 mmHg, clearly illustrating in which patients PP can be proposed. Our population of 57 patients positioned prone was very close to other series in terms of lung injury, as described above, but also in terms of hemodynamic impairment. For instance, 58% of patients required infusion of vasopressor versus 57% in the Australian–New Zealand study [4] and 44% in the recent study by Roch et al. [18]. In the study of Villar et al. [1], only 39% of patients had shock. Finally, the effect of PP on survival may also be related to the combined low stretch ventilation strategy, as explained above, but also to the timing of PP (24–48 h after intubation), which is especially early compared with previous studies.
In conclusion, we demonstrate the clinical feasibility of routine PP in patients with PaO2/FiO2 below 100 mmHg after 24–48 h and suggest that, when combined with a low stretch ventilation strategy, it is protective leading to a high survival rate.
References
Villar J, Pérez-Méndez L, Lopez J, Belda J, Blanco J, Saralegui I, Suarez-Sipmann F, Lopez J, Lubillo S, Kacmarek R, on Behalf of the HELP Network (2007) An early PEEP/FiO2 trial identifies different degrees of lung injury in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 176:795–804
Peek G, Moore H, Moore N, Sosnowski A, Firmin R (1997) Extracorporeal membrane oxygenation for adult respiratory failure. Chest 112:759–764
Peek G, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany M, Hibbert C, Truesdale A, Clemens F, Firmin R, Elbourne D, For the CESAR trial collaboration (2009) Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicenter randomized controlled trial. Lancet 374:1351–1363
The Australian New Zealand extracorporeal membrane oxygenation (ANZ ECMO) influenza investigators (2009) Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA 302:1888–1895
Sud S, Friedrich J, Taccone P, Polli F, Adhikari N, Latini R, Pesenti A, Guerin C, Mancebo J, Curley M, Fernandez R, Chan MC, Beuret P, Voggenreiter G, Sud M, Tognoni G, Gattinoni L (2010) Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med 36:585–599
Le Gall JR, Lemeshow S, Saulnier F (1993) A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Murray J, Matthay M, Luce J, Flick M (1988) An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 138:720–723
Annane D, Aegerter P, Jars-Guincestre MC, Guidet B, CUB-Réa Network (2003) Current epidemiology of septic shock: the CUB-Réa Network. Am J Respir Crit Care Med 168:165–172
Bernard G, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Morris A, Spragg R (1994) The American-European consensus conference of ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149:818–824
Page B, Vieillard-Baron A, Beauchet A, Aegerter P, Prin S, Jardin F (2003) Low stretch ventilation strategy in acute respiratory distress syndrome: eight years of clinical experience in a single center. Crit Care Med 31:765–769
Vieillard-Baron A, Prin S, Schmitt JM, Augarde R, Page B, Beauchet A, Jardin F (2002) Pressure-volume curves in acute respiratory distress syndrome. Clinical demonstration of the influence of expiratory flow limitation on the initial slope. Am J Respir Crit Care Med 165:1107–1112
Prin S, Chergui K, Augarde R, Page B, Jardin F, Vieillard-Baron A (2002) Ability and safety of heated humidifier to control hypercapnic acidosis in severe ARDS. Intensive Care Med 28:1756–1760
Douglas WW, Rehder K, Beynen FM, Sessler AD, Marsh HM (1977) Improved oxygenation in patients with acute respiratory failure: the prone position. Am Rev Respir Dis 115:559–566
Guerin C, Gaillard S, Lemasson S, Ayzac L, Girard R, Beuret P, Palmier B, Le QV, Sirodot M, Rosselli S, Cadiergue V, Sainty JM, Barbe P, Combourieu E, Debatty D, Rouffineau J, Ezingeard E, Millet O, Guelon D, Rodriguez L, Martin O, Renault A, Sibille JP, Kaidomar M (2004) Effects of systematic prone positioning in hypoxemic acute respiratory failure: a randomized controlled trial. JAMA 292:2379–2387
Gattinoni L, Tognoni G, Pesenti A, Taccone P, Mascheroni D, Labarta V, Malacrida R, Di Giulio P, Fumagalli R, Pelosi P, Brazzi L, Latini R (2001) Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med 345:568–573
Mancebo J, Fernandez R, Blanch L, Rialp G, Gordo F, Ferrer M, Rodriguez F, Garro P, Ricart P, Vallverdu I, Gich I, Castano J, Saura P, Dominguez G, Bonet A, Albert RK (2006) A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med 173:1233–1239
Chiumello D, Cressoni M, Racagni M, Landi L, Li Bassi G, Polli F, Carlesso E, Gattinoni L (2006) Effects of thoraco-pelvic supports during prone position in patients with acute lung injury/acute respiratory distress syndrome: a physiological study. Crit Care 10:R87
Roch A, Lepaul-Ercole R, Grisoli D, Bessereau J, Brissy O, Castanier M, Dizier S, Forel JM, Guervilly C, Gariboldi V, Collart F, Michelet P, Perrin G, Charrel R, Papazian L (2010) Extracorporeal membrane oxygenation for severe influenza A (H1N1) acute respiratory distress syndrome: a prospective observational comparative study. Intensive Care Med 36:1899–1905
Vieillard-Baron Rabiller A, Chergui K, Peyrouset O, Page B, Beauchet A, Jardin F (2005) Prone position improves mechanics and alveolar ventilation in acute respiratory distress syndrome. Intensive Care Med 31:220–226
Villar J, Pérez-Méndez, Kacmarek R (1999) Current definitions of acute lung injury and the acute respiratory distress syndrome do not reflect their true severity and outcome. Intensive Care Med 25:930–935
Tacccone P, Pesenti A, Latini R, Polli F, Vagginelli F, Mietto C, Caspani L, Raimondi F, Bordone G, Iapichino G, Mancebo J, Guerin C, Ayzac L, Blanch L, Fumagalli R, Tognoni G, Gattinoni L, For the Prone-Supine II study group (2009) Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA 302:1977–1984
Estenssoro E, Dubin A, Laffaire E, Canales HS, Saenz G, Moseinco M, Bachetti P (2003) Impact of positive end-expiratory pressure on the definition of acute respiratory distress syndrome. Intensive Care Med 29:1936–1942
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Charron, C., Bouferrache, K., Caille, V. et al. Routine prone positioning in patients with severe ARDS: feasibility and impact on prognosis. Intensive Care Med 37, 785–790 (2011). https://doi.org/10.1007/s00134-011-2180-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2180-x