Abstract
Obstructive sleep apnea syndrome (OSAS) is an independent risk factor for atherosclerosis and arterial thrombosis, which are associated with high cardiovascular (CV) morbidity and mortality. In studies performed in clinical populations with elevated CV event risk profiles, the occurrence of moderate to severe OSAS was very often accompanied by a worsened vascular function and increased prevalence of structural abnormalities. Recent investigations of atherosclerosis in OSAS have focused on thrombotic tendency and blood viscosity, providing new insight into mechanisms of the disease. Despite that knowledge about the mechanisms of development of CV disease in patients with OSAS is still incomplete, observations confirm a relationship between sleep-disordered breathing and the rheological properties (flow properties) of blood. While platelet dysfunction and hypercoagulability (PDMPs, PaI-1, and SF) play important roles in the pathogenesis of vascular disease, there are limited studies on the potential role of blood viscosity in the development of vascular disease in OSAS.
Similar content being viewed by others
Introduction
Obstructive sleep apnea syndrome (OSAS) is characterized by recurrent episodes of complete or partial collapse of the upper airway during sleep, resulting in apneas or hypopneas, respectively. Repetitive episodes of obstruction cause intermittent drops in blood oxygen and increases in carbon dioxide levels, which can lead to frequent arousals from sleep [1]. The most important complications are cardiovascular (CV) disturbances, resulting in severe morbidity and mortality [2]. Increased blood clotting, caused by changes in the rheological properties (flow properties) of blood and plasma, seems to be an important factor linking OSAS and CV complications [3].
Atherosclerosis is a chronic inflammatory disease that has a silent course for a few decades before reaching clinical significance. Atherosclerosis is common in OSAS patients, and the majority of studies have demonstrated an excess of mortality in OSAS which is associated with the severity of the atherosclerosis [4, 5]. One study [6] showed that the incidence of CV events, e.g., stroke, myocardial infarction, and CV death, is elevated in OSAS patients and that the odds ratio (OR) of CV events or mortality varies from two to seven orders of magnitude for moderate to severe OSAS. This finding was confirmed by a subsequent study [7] of 202 consecutive patients who were investigated with electron beam computed tomography. Patients were asymptomatic with regard to coronary artery disease (CAD) and were investigated with an overnight sleep study (OSAS prevalence 76 %). Coronary artery calcification (CAC) was present in 67 % of those with OSAS and in 31 % of those without OSAS (p ≤ 0.001). Median CAC score was nine in OSA patients and zero in non-OSAS patients (p ≤ 0.001), strongly supporting the theory that OSAS is an independent risk factor for CAC.
This review provides a critical analysis of the current evidence for an association between OSAS and hemostatic alterations, blood viscosity, and atherosclerosis, and discusses basic mechanisms that may be responsible for this association. Suggestions are also made for future research.
Blood Viscosity: The Important Parameter in Cardiovascular Disease
Blood viscosity is defined as the inherent resistance of blood to flow and is a highly dynamic property. It is a direct measure of the ability of blood to flow through the vessels. This is in contrast to hematocrit, lipid profiles, glucose, creatinine, and plasma protein concentrations, among others, which are relatively static at any given moment in time. Normal adult blood viscosity is 40/100 and reported in units of millipoise (mP) [8], which is convenient for reporting blood pressure and other vital signs. It is the critical biophysical parameter that determines how much friction the blood causes against the vessels.
It is difficult to test both systolic and diastolic blood viscosity for a single patient, as we do for blood pressure. Increased blood viscosity is the only biological parameter that has been linked with major CV risk factors, including high blood pressure, elevated low-density lipoprotein (LDL) cholesterol, low high-density lipoprotein (HDL) cholesterol, type II diabetes, metabolic syndrome, obesity, smoking, age, and male gender. Importantly, high blood viscosity is easily modifiable with safe lifestyle-based interventions [9].
Reduced Artery Flow as Reduced Artery Function in Non-Obstructive Sleep Apnea Syndrome Populations
The relationship between blood viscosity and blood flow (or perfusion) can be an intuitive concept but difficult to show in the clinic. Clinically, measurement of blood viscosity could be a useful surrogate measure of perfusion and much better suited than perfusion studies for routine use in the general population. Simply put, elevated blood viscosity translates directly into reduced blood perfusion. When blood flow is sufficiently disturbed, hyperviscosity can present as organ dysfunction with or without the sensation of pain. Increased viscosity may be the reason why the other biochemical, metabolic factors are important and is the underlying mechanism by which these other factors convey the preinflammatory insult to the arterial walls.
The largest and longest-running prospective study on blood viscosity, called the Edinburgh Artery Study, published a report on cognitive decline in 2010. Blood viscosity was predictive of cognitive decline over a 4-year period in 452 elderly subjects (p < 0.05). The researchers tested viscosity in a random population of adults. Ten years later and again 14 years later they ran cognitive tests to assess cognitive decline in the study cohort over the 4-year interim period. Four different tests were used to assess logical memory, nonverbal reasoning, verbal fluency, and cognitive processing speed. A general cognitive factor was also computed to represent the variance common to all four cognitive tests. After controlling for age, higher blood viscosity correlated with lower scores on the general cognitive factor (p < 0.01) and all of the individual cognitive tests (p < 0.05 to < 0.01), with the exception of the verbal fluency test [10].
In a prospective study [11], 331 middle-aged men with high blood pressure were followed for up to 12 years after measuring diastolic blood viscosity (i.e., low shear rate viscosity). The patients were divided into three groups according to viscosity levels: those in the highest tertile had more than three times more CV events than those in the lowest tertile (hazard ratio [HR] = 3.42, 95 % confidence interval [CI] = 1.4–8.4, p = 0.006). In regard to atherogenesis, blood viscosity correlated with the extent of arterial stenoses in arteries supplying the lower limbs, as measured by the ankle-brachial pressure index (ABPI); plasma fibrinogen also showed an independent association with the ABPI [12].
In regard to thrombogenesis [13], all major CV risk markers were associated with an imbalance of blood coagulation over fibrinolysis, as measured by plasma levels of thrombin and plasmin degradation products of fibrinogen. Besides, smokers have reversible increases in blood viscosity, hematocrit, polycythemia, and plasma viscosity compared with nonsmokers; the increase in plasma viscosity is largely a result of hyperfibrinogenemia, because serum viscosity does not increase significantly [13]. The first clear epidemiologic data [14], from the Northwick Park Heart Study, showed that initial fibrinogen levels were associated with CV mortality, independent of other risk factors. Finally, fibrinogen has been shown to be a strong risk marker for ischemic heart disease and stroke in several other studies and to correlate with most major CV risk markers in both sexes. Fibrinogen and its degradation products may also damage blood vessel walls by stimulating smooth muscle proliferation and migration [15].
Increased Blood Viscosity and Risk of Cardiovascular Disease in Obstructive Sleep Apnea Syndrome Populations
Obstructive sleep apnea syndrome has increasingly been linked to CV disease. Disturbances in inflammatory and coagulation profiles, particularly those of cytokines, platelets, and fibrinogen, have emerged as possible mediators of CV pathophysiology in OSAS. Blood viscosity is defined, as stated in the preceding section, as the internal resistance of the blood to shear forces. Blood viscosity is determined by plasma viscosity, hematocrit (volume fraction of erythrocytes, which constitute 99.9 % of the cellular elements), and the mechanical behavior of erythrocytes [16]. The latter refers to their aggregation and deformation abilities, with deformation meaning to change shape while maintaining a constant volume [17]. Increased blood clotting, caused by changes in the rheological properties of blood and plasma, seems to be an important factor linking OSAS and CV complications [18]. Although many papers on OSAS and its traditional CV risk factors have been published, few have taken into account the role of blood [19] and the effect of OSAS on the rheological properties of blood coagulation [20]. Furthermore, methodological differences among these studies hinder their comparative analysis. However, the evidence that exists strongly suggests that the viscosity of plasma and whole blood is abnormally high in patients with OSAS.
The work of Chin et al. [21] confirmed increased levels of fibrinogen and hematocrit in 11 patients with OSAS in the morning, suggesting increased blood viscosity. Similarly, Nobili et al. [22] showed that blood viscosity was increased in the mornings in a group of 12 patients with OSAS but not in a group of eight healthy controls. The criterion for diagnosis was an apnea–hypopnea index (AHI) >5 h/s confirmed by a polysomnography study and the presence of clinical symptoms.
Reinhart et al. [23] described the effect of 6 months of continuous positive airway pressure (CPAP) therapy on a group of 13 patients with OSAS and compared them to eight controls. They found that morning plasma viscosity was higher in patients with OSAS and that fibrinogen levels showed a correlation with plasma viscosity. The treatment with CPAP normalized the blood viscosity and platelet activity. They also confirmed that blood viscosity and platelet activity in patients treated with CPAP were comparable to those of healthy controls, suggesting that short-term CPAP therapy is effective in reducing blood viscosity in OSAS patients.
A study by Zhang et al. [24] reported similar results. The biochemical characteristics of a prothrombotic state were evaluated in a group of 41 OSAS patients (mean age = 63.4 ± 4 years) before and after 30 days of CPAP therapy and compared the results to those of healthy, age-matched controls. Before CPAP therapy, they confirmed a statistically significant increase in hematocrit, blood viscosity, and platelet aggregation. The 30-day CPAP therapy resulted in morning reduction of hematocrit, total blood viscosity, platelet aggregation, and prolongation of the prothrombin state.
Tazbirek et al. [20] found an increase in blood viscosity and erythrocyte aggregation in obese men with OSAS compared to patients without OSAS, matched in terms of age, body mass index (BMI), and the incidence of coexisting diseases. They showed that there was a correlation between the values of measured parameters before the start of CPAP therapy (blood viscosity, corrected blood viscosity, plasma viscosity, aggregation index) in patients with severe OSAS. Dikmenoğlu et al. [16] compared the rheological properties of blood in 11 patients with severe OSAS to a control group matched for age and gender. They found that plasma viscosity in patients with severe OSAS was significantly higher.
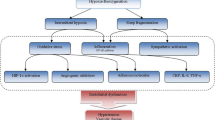
There are currently several theories to explain the increase in the viscosity of plasma and whole blood observed in OSAS patients: changes in connections between plasma bridging proteins, increased plasma fibrinogen levels, and excessive sympathetic nervous system activity leading to increased platelet aggregation.
Increased viscosity affects the frictional forces that inhibit the movement of erythrocytes; it positively correlates with total cholesterol [25], LDLs, and triglycerides [26]. The authors observed positive linear correlations of viscosity with total cholesterol and with apoproteins A2 and B. These associations were moderately strong, persisted after adjustments for confounding variables, and are consistent with the in vitro effects of lipoproteins on viscosity.
Another theory to explain the increase in the viscosity of plasma and whole blood in OSAS patients is increased levels of fibrinogen. Correlations between increased levels of fibrinogen and plasma viscosity in obese patients [27], as well as between fibrinogen and ischemic heart disease, have been confirmed [28]. Wessendorf et al. [29] showed that elevated plasma fibrinogen levels in stroke patients are accompanied by a higher incidence of OSAS. Their data confirmed a positive correlation between the plasma fibrinogen concentration and the number and duration of recorded breathing disorders during sleep, and a negative correlation with minimum and average blood saturation. The authors suggest that high concentrations of fibrinogen may lead to an increased incidence of vascular disease in OSAS.
Another important association between OSAS and CV disease is excessive sympathetic nervous system activity. Increased platelet aggregation in patients with OSAS may be secondary to the increased nocturnal levels of sympathetic activity and catecholamines. Hypoxia, hypercapnia, and blood pressure increases associated with OSAS are elements of endothelial function impairment. Increased levels of endothelin may contribute to vasoconstriction and other changes in the CV system with an increase in plasma viscosity and hematocrit and reduction in vessel cross-section [30].
Finally, systemic inflammation plays an important role in the development of atherosclerosis. The pathogenesis of inflammation and atherosclerosis in OSAS patients has not been well-defined. The desaturation–reoxygenation sequence is a typical pattern coupled with the majority of respiratory events. This sequence, defining intermittent hypoxia (IH), leads to oxidative stress with production of reactive oxygen species (ROS). The increased levels of ROS contribute to the generation of adhesion molecules, activation of leukocytes, and production of systemic inflammation. These mechanisms cause vascular endothelial damage and coagulation dysfunction [31]. Evidence has accumulated over the past decade of an important role for sympathetic nervous system overactivity in the pathophysiology of vascular dysfunction in OSAS. The mechanisms remain unclear, particularly the role of IH and sleep fragmentation and the effects on different types and sizes of blood vessels. Animal models may be most suited to this area of investigation [32].
Detailed Analysis of Blood Coagulation Abnormalities in Obstructive Sleep Apnea Syndrome
Increased CV risk in OSAS patients may also be linked to abnormalities of coagulation and excessive platelet activation; this topic has been recently reviewed [33]. Platelet activation by various agonists such as thrombin, collagen, and inflammatory cytokines, or by physical stimuli such as hypoxia and shear stress, results in the shedding of submicroscopic membrane vesicles known as platelet-derived microparticles (PDMPs) [34]. PDMPs are less than 1.5 μm in diameter and enriched with procoagulant platelet proteins [35]; they have a negatively charged phospholipid surface, allowing them to bind to activated coagulation factors and exposed tissue factors under various conditions. Since PDMPs are formed by platelets upon activation, their membrane possesses all the properties of the activated platelet membrane, including the ability to bind to the components of procoagulant complexes such as factors V (Va) and VIII (VIIIa). Furthermore, the binding site densities of these proteins on PDMP membranes exceed those observed on platelet membranes, such that the procoagulant activity of PDMP membranes is approximately 50–100-fold higher than that of activated platelets [36, 37]. The authors, in summary, reported that plasma PDMPs are increased in patients with acute coronary syndrome, suggesting that PDMPs may play a role in the pathogenesis of arterial thrombosis and atherosclerosis, and in OSAS.
The level of D-dimer, a plasmin-derived degradation product of cross-linked fibrin, is a direct measure of activated coagulation and has been used as a measure of hypercoagulability [38]. Interestingly, two groups have recently reported an increased D-dimer level in untreated OSAS and its correlation with the severity of nocturnal hypoxemia, suggesting that a hypercoagulable state is potentially involved in CV risk in OSAS patients [39, 40]. Other investigators have found increased fibrinogen levels in both adults and children with sleep-disordered breathing (SDB) [19, 41] and evidence of platelet activation, which decreased after CPAP treatment [42, 43].
Increased levels of the procoagulation factors soluble CD40 ligand (sCD40L) and soluble P-selectin (sP-selectin) have been found in some OSAS patients; levels of sCD40L and sP-selectin correlated with the degree of nocturnal hypoxic stress and arousals and were reduced by CPAP therapy during sleep [44]. sCD40L and sP-selectin appear in plasma during the early stage of blood coagulation and are well-known indicators of thrombogenic conditions such as disseminated intravascular coagulation (DIC) [45]. One study demonstrated that sCD40L concentrations predict future risk of myocardial infarction and stroke [46]. sP-selectin is involved in leukocyte rolling and attachment and thus can play an important role in the initiation of atherosclerosis [47]. In addition, levels of both sCD40L and sP-selectin are increased in patients with hypertension, hyperlipidemia, and diabetes mellitus [48].
In contrast, elevated levels of an anticoagulation factor that inhibits the formation of fibrin strands, and therefore inhibits blood coagulation, were found in one study of OSAS patients. Plasminogen activator-1 (PAI-1), a member of the serine protease inhibitor family, inhibits fibrinolytic activity by binding to tissue-type plasminogen activator (tPA). It was demonstrated that a higher AHI (events per hour of sleep) and nadir nocturnal SaO2 (Nadir SaO2%) were both associated with a higher concentration of circulating PAI-1 in a group of OSAS patients [49]. Although this evidence is not consistent with the idea that OSAS results in increased risk of CV disease via increased blood coagulation, increased concentrations of PAI-1 predict the occurrence of a first acute myocardial infarction in middle-aged men and women with a high prevalence of coronary heart disease [50]. In short, many uncertainties still remain as to the independent effects of OSAS on increased blood coagulability, largely due to the common coexistence of other CV risk factors [48] and the incomplete normalization of coagulation after CPAP treatment [51].
These same pathophysiological derangements are prothrombotic and could promote the development of venous thromboembolic disease. Several previous case reports and uncontrolled cohort studies have suggested a possible association between OSAS and pulmonary embolism (PE) [43, 52]. A recent prospective cross-sectional study determined the prevalence of snoring and risk of OSAS in patients with acute PE. The patients with PE had a significantly higher prevalence of snoring (75 vs. 50 %, OR = 2.91, 95 % CI = 1.60–5.33, p = 0.001) and an increased risk of having OSAS, as defined by the Berlin Questionnaire (65 vs. 36 %, OR = 3.25, 95 % CI = 1.84–5.72, p < 0.001), compared with patients in whom PE was suspected but ruled out. The authors concluded that this association might be independent of other risks factors common to both OSAS and PE. Therefore, OSAS may represent a risk factor for the development of PE [53].
Disruption in Temporal Regulation of Components of the Hemostatic System in Obstructive Sleep Apnea Syndrome
The hematopoietic and blood cell systems and all their components are characterized by a multifrequency time structure with prominent rhythms in cell proliferation and cell function in the circadian and infradian frequency ranges. Some of these rhythms show large enough amplitudes to be clinically important. Rhythm disturbances characterize hematologic and related disease states, e.g., infection and atherosclerosis. Circadian rhythms in the ability to aggregate and in adhesiveness of blood platelets contribute to the transient state of the hypercoagulability. The rhythmic, and thus in their timing to a certain degree, predictable changes in responsiveness of the hematopoietic factors provide an opportunity to improve the effects of growth factors and cytokines and decrease their undesirable side effects [54].
Modern functional genomics approaches may facilitate a better understanding of the molecular events involved in tissue pathogenesis and allow the identification of molecular signatures and pathways. Circadian clock genes use transcriptional–translation feedback loops to control circadian rhythms. Recent studies have demonstrated that expression of some circadian clock genes displays daily oscillation in peripheral tissue, including peripheral blood. Circadian rhythms regulate various functions of the human body and alteration of them has been associated with some diseases [55].
One recent study [56] demonstrated that diurnal variations exist in various hemostatic factors (factor VII, von Willebrand factor, PAI-1) and markers of endothelial dysfunction [asymmetric dimethylarginine (ADMA) and sCD40L], which are involved in the development of CV disease. It therefore seems possible that disruptions in sleep patterns in OSAS patients could result in abnormal temporal regulation of these factors, with corresponding effects on blood coagulation. However, the study showed that day–night variations in levels of factor VII: Ag, von Willebrand factor: Ag, PAI-1, sCD40L, and ADMA are not significantly different between patients with OSAS and controls without OSAS. This suggests that sleep apnea does not have any direct effect on the oscillations of these hemostatic factors. Nevertheless, compared with controls, patients with OSAS showed a higher variability in mean levels of PAI-1 at different times. It is therefore likely that day–night variations in hemostatic factors such as PAI-1 are dependent on the obesity index or metabolic dysfunction rather than on sleep apnea alone; this is an area worthy of investigation in the future.
Conclusion
The basic mechanisms involved in the pathogenesis of CV disease in OSAS are complex and some aspects, particularly those related to the molecular mechanisms of IH, may be more suited to cell culture and/or animal models. Future animal studies will help clarify the interactions between putative OSAS genes and disruption of CV regulation and/or the role of sympathetic excitation or the effect IH on vascular tissue. Large, randomized studies will be necessary to establish fully whether the treatment of OSAS can stop or even reverse atherosclerosis progression and ultimately reduce CV disease.
Further studies are required to establish the practical value of viscosity measurement in risk prediction, the effects of viscosity changes on biological processes relevant to ischemia and IH, and whether long-term reduction in viscosity reduces CV risk. The potential effects of OSAS on circadian variability sleep structure, sleep fragmentation and sleep deprivation have received little attention and also deserve future investigation.
References
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S (1993) The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328:1230–1235
Marin JM, Carrizo SJ, Vicente E, Agusti AG (2005) Long-term cardiovascular outcomes in men with obstructive sleep apnoea–hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365:1046–1053
Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A et al (2008) Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 118:1080–1111
Minoguchi K, Yokoe T, Tazaki T, Minoguchi H, Tanaka A, Oda N, Okada S, Ohta S, Naito H, Adachi M (2005) Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med 172:625–630
Ip MS, Tse HF, Lam B, Tsang KW, Lam WK (2004) Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med 169:348–353
Buchner NJ, Sanner BM, Borgel J, Rump LC (2007) Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am J Respir Crit Care Med 176:1274–1280
Sorajja D, Gami AS, Somers VK, Behrenbeck TR, Garcia-Touchard A, Lopez-Jimenez F (2008) Independent association between obstructive sleep apnea and subclinical coronary artery disease. Chest 133:927–929
Baskurt OK, Hardeman MR, Rampling MW, Meiselman HJ (2007) Handbook of Hemorheology and Hemodynamics. IOS, Lansdale
Holsworth RE, Wright JV (2012) Blood viscosity: the unifying parameter in cardiovascular disease risk. Holist Prim Ore 13:1
Rafnsson S, Deary IJ, Whiteman MC, Rumley A, Lowe GD, Fowkes FG (2010) Haemorheological predictors of cognitive decline: the Edinburgh Artery Study. Age Ageing 39:217–222
Ciuffetti G, Schillaci G, Lombardini R, Pirro M, Vaudo G, Mannarino E (2005) Prognostic impact of low-shear whole blood viscosity in hypertensive men. Eur J Clin Invest 35:93–98
Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ (1991) Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol 20:384–392
Lowe GDO, Wood DA, Douglas JT, Riemersma RA, Mcintyre CCA, Takase T, Tuddenham EGD, Forbes CD, Elton RA, Oliver MF (1991) Relationships of plasma viscosity, coagulation and fibrinolysis to coronary risk factors and angina. Thromb Haemost 65:339–343
Meade TW, North WR, Chakrabarti R, Stirling Y, Haines AP, Thompson SG, Brozovié M (1980) Haemostatic function and cardiovascular death: early results of a prospective study. Lancet 1:1050–1054
Lee AJ, Smith WC, Lowe GD, Tunstall-Pedoe H (1990) Plasma fibrinogen and coronary risk factors: The Scottish Heart Health Study. J Clin Epidemiol 43:913–919
Dikmenoğlu N, Ciftçi B, Ileri E, Güven SF, Seringeç N, Aksoy Y, Ercil D (2006) Erythrocyte deformability, plasma viscosity and oxidative status in patients with severe obstructive sleep apnea syndrome. Sleep Med 7:255–261
Landgraf H (1999) Correlation between plasma viscosity and tissue oxygen tension. Clin Hemorheol Microcirc 20:37–40
Parish JM, Somers VK (2004) Obstructive sleep apnea and cardiovascular disease. Mayo Clin Proc 79:1036–1046
Steiner S, Jax T, Evers S, Hennersdorf M, Schwalen A, Strauer BE (2005) Altered blood rheology in obstructive sleep apnea as a mediator of cardiovascular risk. Cardiology 104:92–96
Tazbirek M, Slowińska L, Skoczyński S, Pierzchała W (2009) Short-term continuous positive airway pressure therapy reverses the pathological influence of obstructive sleep apnea on blood rheology parameters. Clin Hemorheol Microcirc 41:241–249
Chin K, Ohi M, Kita H, Noguchi T, Otsuka N, Tsuboi T, Mishima M, Kuno K (1996) Effects of NCPAP therapy on fibrinogen levels in obstructive sleep apnea syndrome. Am J Respir Crit Care Med 153:1972–1976
Nobili L, Schiavi G, Bozano E, De Carli F, Ferrillo F, Nobili F (2000) Morning increase of whole blood viscosity in obstructive sleep apnea syndrome. Clin Hemorheol Microcirc 22:21–27
Reinhart WH, Oswald J, Walter R, Kuhn M (2002) Blood viscosity and platelet function in patients with obstructive sleep apnea syndrome treated with nasal continuous positive airway pressure. Clin Hemorheol Microcirc 27:201–207
Zhang X, Yin K, Wang H, Su M, Yang Y (2003) Effect of continuous positive airway pressure treatment on elderly Chinese patients with obstructive sleep apnea in the prothrombotic state. Chin Med J (Engl) 116:1426–1428
Lowe GD (1992) Blood viscosity, lipoproteins and cardiovascular risk. Circulation 85:2329–2331
De Simone G, Devereux RB, Chien S, Alderman MH, Atlas SA, Larach JH (1990) Relation of blood viscosity to demographic and physiologic variables and to cardiovascular risk factors in apparently normal adults. Circulation 81:107–117
Fanari P, Somazzi R, Nasrawi F, Ticozzelli P, Grugni G, Agosti R, Longhini E (1993) Haemorheological changes in obese adolescents after short-term diet. Int J Obes Relat Metab Disord 17:487–494
Heinrich J, Balleisen L, Schulte H, Assmann G, van de Loo J (1994) Fibrinogen and factor VII in the prediction of coronary risk. Results from the PROCAM study in healthy men. Arterioscler Thromb 14:54–59
Wessendorf TE, Thilmann AF, Wang YM, Schreiber A, Konietzko N, Teschler H (2000) Fibrinogen levels and obstructive sleep apnea in ischemic stroke. Am J Respir Crit Care Med 162:2039–2042
McNicholas WT, Bonsignore, The Management Committee of EU COST ACTION B26 (2007) Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanism and research priorities. Eur Respir J 29:156–178
Lavie L (2003) Obstructive sleep apnoea syndrome—an oxidative stress disorder. Sleep Med Rev 7:35–51
Nieto FJ, Herrington DM, Redline S, Benjamin EJ, Robbins JA (2004) Sleep apnea and markers of vascular endothelial function in a large community sample of older adults. Am J Respir Crit Care Med 169:354–360
von Känel R, Dimsdale JE (2003) Hemostatic alterations in patients with obstructive sleep apnea and the implications for cardiovascular disease. Chest 124:1956–1967
Mackman N (2009) On the trail of microparticles. Circ Res 104:925–927
Nomura S (2001) Function and clinical significance of platelet-derived microparticles. Int J Hematol 74:397–404
Sinauridze EI, Kireev DA, Popenko NY, Pichugin AV, Panteleev MA, Krymskaya OV, Ataullakhanov FI (2007) Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost 97(3):425–434
Gilbert GE, Sims PJ, Wiedmer T, Furie B, Furie BC, Shattil SJ (1991) Platelet-derived microparticles express high affinity receptors for factor VIII. J Biol Chem 266:17261–17268
Dougu N, Takashima S, Sasahara E, Taguchi Y, Toyoda S, Hirai T, Nozawa T, Tanaka K, Inoue H (2011) Predictors of poor outcome in patients with acute cerebral infarction. J Clin Neurol 7(4):197–202
Shitrit D, Peled N, Shitrit AB, Meidan S, Bendayan D, Sahar G, Kramer MR (2005) An association between oxygen desaturation and D-dimer in patients with obstructive sleep apnea syndrome. Thromb Haemost 94:544–547
von Kanel R, Loredo JS, Powell FL, Adler KA, Dimsdale JE (2005) Short-term isocapnic hypoxia and coagulation activation in patients with sleep apnea. Clin Hemorheol Microcirc 33:369–377
Kaditis AG, Alexopoulos EI, Kalampouka E, Kostadima E, Angelopoulos N, Germenis A, Zintzaras E, Gourgoulianis K (2004) Morning levels of fibrinogen in children with sleep disordered breathing. Eur Respir J 24:790–797
Shimizu M, Kamio K, Haida M, Ono Y, Miyachi H, Yamamoto M, Shinohara Y, Ando Y (2002) Platelet activation in patients with obstructive sleep apnea syndrome and effects of nasal-continuous positive airway pressure. Tokai J Exp Clin Med 27:107–112
Hui DS, Ko FW, Fok JP, Chan MC, Li TS, Tomlinson B, Cheng G (2004) The effects of nasal continuous positive airway pressure on platelet activation in obstructive sleep apnea syndrome. Chest 125:1768–1775
Geiser T, Buck F, Meyer BJ, Bassetti C, Haeberli A, Gugger M (2002) In vivo platelet activation is increased during sleep in patients with obstructive sleep apnea syndrome. Respiration 69:229–234
Siegal T, Seligsohn U, Aghai E, Modan M (1978) Clinical and laboratory aspects of disseminated intravascular coagulation (DIC): a study of 118 cases. Thromb Haemost 39:122–134
Plaikner M, Peer A, Falkensammer G, Schmidauer C, Pechlaner C, Griesmacher A, Pachinger O, Mair J (2009) Lack of association of soluble CD40 ligand with the presence of acute myocardial infarction or ischemic stroke in the emergency department. Clin Chem 55(1):175–178
Jacobin-Valat MJ, Deramchia K, Mornet S, Hagemeyer CE, Bonetto S, Robert R, Biran M, Massot P, Miraux S, Sanchez S, Bouzier-Sore AK, Franconi JM, Duguet E, Clofent-Sanchez G (2010) MRI of inducible P-selectin expression in human activated platelets involved in the early stages of atherosclerosis. NMR Biomed 24:413–424
Robinson GV, Pepperell JC, Segal HC, Davies RJ, Stradling JR (2004) Circulating cardiovascular risk factors in obstructive sleep apnea: data from randomised controlled trial. Thorax 59:777–782
Ryan S, Taylor CT, McNicholas WT (2009) Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnea syndrome? Thorax 64:631–636
Maruyama K, Morishita E, Sekiya A, Omote M, Kadono T, Asakura H, Hashimoto M, Kobayashi M, Nakatsumi Y, Takada S, Ohtake S (2012) Plasma levels of platelet-derived microparticles in patients with obstructive sleep apnea. J Atheroscler Thromb 19(1):98–104
Arnulf I, Merino-Andreu M, Perrier A, Birolleau S, Similowski T, Derenne JP (2002) Obstructive sleep apnea and venous thromboembolism. JAMA 287:2655–2656
Ambrosetti M, Lucioni A, Ageno W, Conti S, Neri M (2004) Is venous thromboembolism more frequent in patients with obstructive sleep apnea syndrome? J Thromb Haemost 2:1858–1860
Epstein MD, Segal LN, Ibrahim SM, Friedman N, Bustami R (2010) Snoring and the risk of obstructive sleep apnea in patients with pulmonary embolism. Sleep 33:1069–1074
Haus E (1996) Biologic rhythms in hematology. Patol Biol (Paris) 44:618–630
Lamont EW, James FO, Boivin DB, Cermakian N (2007) From circadian clock gene expression to pathologies. Sleep Med 8:547–556
Barceló A, Piérola J, de la Peña M, Esquinas C, Sanchez-de la Torre M, Ayllón O, Alonso A, Agusti AG, Barbè F (2011) Day-night variations in endothelial dysfunction markers and haemostatic factors in sleep apnoea. Eur Respir J 39:913–918
Acknowledgments
The authors thank Miss Stephanie Grice MBiochem, MSc (Oxon), from the UK, for the editing of this manuscript.
Conflict of interest
The authors have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toraldo, D.M., Peverini, F., De Benedetto, M. et al. Obstructive Sleep Apnea Syndrome: Blood Viscosity, Blood Coagulation Abnormalities, and Early Atherosclerosis. Lung 191, 1–7 (2013). https://doi.org/10.1007/s00408-012-9427-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-012-9427-3