Abstract
Background
Diaphragm movement is essential for adequate ventilation, and when the diaphragm is adversely affected patients face lifelong positive-pressure mechanical ventilation or death. This report summarizes the complete worldwide multicenter experience with diaphragm pacing stimulation (DPS) to maintain and provide diaphragm function in ventilator-dependent spinal cord injury (SCI) patients and respiratory-compromised patients with amyotrophic lateral sclerosis (ALS). It will highlight the surgical experiences and the differences in diaphragm function in these two groups of patients.
Methods
In prospective Food and Drug Administration (FDA) trials, patients underwent laparoscopic diaphragm motor point mapping with intramuscular electrode implantation. Stimulation of the electrodes ensued to condition and strengthen the diaphragm.
Results
From March of 2000 to September of 2007, a total of 88 patients (50 SCI and 38 ALS) were implanted with DPS at five sites. Patient age ranged from 18 to 74 years. Time from SCI to implantation ranged from 3 months to 27 years. In 87 patients the diaphragm motor point was mapped with successful implantation of electrodes with the only failure the second SCI patient who had a false-positive phrenic nerve study. Patients with ALS had much weaker diaphragms identified surgically, requiring trains of stimulation during mapping to identify the motor point at times. There was no perioperative mortality even in ALS patients with forced vital capacity (FVC) below 50% predicted. There was no cardiac involvement from diaphragm pacing even when analyzed in ten patients who had pre-existing cardiac pacemakers. No infections occurred even with simultaneous gastrostomy tube placements for ALS patients. In the SCI patients 96% were able to use DPS to provide ventilation replacing their mechanical ventilators and in the ALS studies patients have been able to delay the need for mechanical ventilation up to 24 months.
Conclusion
This multicenter experience has shown that laparoscopic diaphragm motor point mapping, electrode implantation, and pacing can be safely performed both in SCI and in ALS. In SCI patients it allows freedom from ventilator and in ALS patients it delays the need for ventilators, increasing survival.
Similar content being viewed by others
Diaphragm pacing stimulation (DPS) is designed to replace or delay the need for patients requiring long-term positive-pressure mechanical ventilation via tracheostomies. Ventilators, although life saving, have a myriad of complications that include: difficulty with speech, increased secretions and need for suctioning, loss of sense of smell, constant noise, difficulty with transfers, and inability to find living facilities or nursing care, and the cost of being on a ventilator approaches US $200,000 a year. Also mechanical ventilation greatly decreases life expectancy, primarily due to respiratory infections, because of the inherent poor posterior lobe ventilation while on ventilators. DPS provides natural negative-pressure ventilation with the patient’s own diaphragm and overcomes many of these issues.
Of 11,000 new spinal cord injured (SCI) patients each year in the USA, slightly more than one-half are affected by quadriplegia, with 4% requiring long-term mechanical ventilation. The standard therapy for high-level spinal-cord-injured patients is mechanical ventilation via tracheostomy. Yet this treatment is not without harm. Among patients with spinal cord injury at similar levels, the need for mechanical ventilation decreases survival rates from 84% in the nonventilated group to only 33% in the ventilated group [1]. The major cause of mortality in patients with amyotrophic lateral sclerosis (ALS or Lou Gehrig’s disease) is respiratory insufficiency because of the progressive decline of 3–5% per month of their forced vital capacity (FVC) due to the progressive loss of motor neurons leading to respiratory muscle weakness. In this group of patients instead of replacing mechanical ventilation the goal is to delay the need for it by implanting DPS and stimulating the muscle to maintain diaphragm strength prior to the end-stage weakness.
Repair of the injured spinal cord or arresting motor neuron death in ALS by regeneration therapy or perhaps stem cell therapy remains a lofty but elusive goal. Until that goal of a cure is realized techniques have to be developed that can increase the quality of life of SCI patients by removing them from ventilators or increasing the quality and life span of ALS patients by delaying the choice of death or mechanical ventilation. Ragnarssson elegantly outlined in the Sir Ludwig Guttmann Lecture the history and future directions of how functional electrical stimulation (FES) can improve the quality of life of patients with spinal cord injury [2]. Researchers at University Hospitals Case Medical Center and Case Western Reserve University designed a FES system for the diaphragm that has become the present diaphragm pacing stimulation (DPS) system (NeuRx RA/4 system, Synapse Biomedical, Oberlin, OH, USA). This system was designed after a series of animal and preliminary human investigations and the data can be reviewed through previous publications [3–8].
The initial SCI and ALS patients have been previously reported and the long-term outcomes are in pending publications [9–14]. The objective of this paper is to report on the entire operative worldwide experience of the DPS system for both SCI and ALS patients which has not been reported. It will highlight the differences in these two groups of patients: the SCI group, in which patients have completely intact spinal motor neurons and diaphragm but disruption of the signal pathway from the upper respiratory center, and the ALS group, in which patients progressively lose both upper and lower motor neurons.
Methods
This is a prospective evaluation of all patients implanted with the DPS system from 3/2000 until 9/2007. This study was undertaken under FDA Investigational Device Exemption (IDE) G920162 for spinal-cord-injured patients and G040142 for patients with amyotrophic lateral sclerosis. Each site’s Institutional Review Board (IRB) approved the study. The studies were registered at clinicaltrials.gov with the specific identifiers NCT00010374 and NCT00420719. Informed consent was obtained by each participant per IRB and FDA guidelines. All patient demographics, operative experiences, and perioperative results were recorded prospectively.
In the SCI trial, the primary inclusion criterion for DPS implantation was a chronic ventilator-dependent high-level SCI patient with a stimulatable diaphragm (intact phrenic nerves). The primary endpoint for demonstration of probable benefit was the ability of the DPS system to provide clinically acceptable tidal volume for at least four continuous hours of pacing. For a male patient, the tidal volume required to meet basal metabolism is defined as 7 ml/kg body weight. The tidal volume is defined as 6 ml/kg body weight for females. There was an additional IRB protocol with a supplement to the FDA to include an additional study arm to investigate subjects with cardiac pacemaker. Cardiac pacemakers are frequently utilized to help spinal-cord-injured patients who develop bradycardia after their injury.
The ALS patients being reported were involved in three separate IRB trials under the same IDE with some overlap of the trials. One of the primary inclusion criteria was FVC above 50% predicted at enrollment and 45% at implantation because of the concern for taking a patient with severe respiratory dysfunction to surgery. The first group, involving 16 patients, was a safety and feasibility trial to confirm that implantation and stimulation would not have any adverse effects. The second group of two patients had low FVC and did not meet the inclusion criteria of being above 50% predicted FVC at the time of enrollment and were implanted under compassionate use. The third group, of 20 patients, was involved in the initial aspect of a multicenter pivotal trial that will eventually consist of 100 patients. Since ALS patients are such a heterogeneous group the trial was designed such that each patient will be their own control. Therefore in the first and third groups of patients, patients were evaluated three times with a battery of tests prior constituting the lead in prior to surgical implantation. After surgical implantation and diaphragm conditioning the patients were followed with the same tests every 4–12 weeks until the 1-year time period ended. The tests over the course of the trial for these two groups of patients included the Short Form 36 (SF-36), Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFR-R) scoring, phrenic nerve studies, diaphragm ultrasound thickness, fluoroscopic sniff tests, pulmonary function tests, arterial blood gases, laboratory tests, and electrode characterizations including electromyographic assessments. The primary endpoint of the pivotal trial is to assess the rate of decline of FVC pre and post implantation of DPS and assess the effect of DPS. Since that is still ongoing this paper will report on the patient characteristics at the time of surgical implantation and assess operative results. In the second group of two patients who were much later in their disease process, the patients were followed remotely by assessing the electromyographic activity of their diaphragm and pulse oximetry utilizing a home polysomnography system (CleveMed, Cleveland, OH). Continuous electromyogram (EMG) was recorded from the implanted diaphragm electrodes.
The DPS surgery has been previously described and consists of diaphragm mapping and electrode implantation [12]. Neuromuscular blocking agents or paralyzing agents cannot be part of the anesthetic management for the procedure. A total of four ports are used. The 12-mm epigastric port is placed after dividing the falciform ligament, which allows easier access of the implant instrument for both diaphragms and unimpeded exit of the pacing electrodes through the epigastric port.
Mapping involves finding the motor point of the diaphragm where stimulation causes the greatest contraction of the diaphragm. The mapping instrument is held onto the diaphragm by suction with stimulation from a clinical station, and both qualitative and quantitative data is obtained from the stimulation (Fig. 1). Quantitatively, changes in abdominal pressures are measured from one of the laparoscopic ports. Qualitatively, visual observation of the diaphragm motion during a stimulated contraction is used to assess the optimal location for lead implantation. The stronger the stimulated contraction, the closer the mapping probe is to the motor point of the diaphragm. Two electrodes are implanted: one at a primary site and one at a secondary site to capture additional diaphragm movement. Electrodes are then implanted using the electrode implant instrument (Fig. 2). The needle at the end of the instrument is placed into the diaphragm muscle and on withdrawal the barb on the end of the electrode releases, allowing the exposed electrode to stay in the muscle. These electrodes along with an anode are tunneled to an appropriate spot on the chest or abdominal wall. If the patient has a cardiac pacemaker, it is simultaneously interrogated while the diaphragm is being paced, to confirm no device-to-device interactions. Testing is performed at nominal and most sensitive sensing settings for the cardiac pacemaker and in sensing modes supported by the cardiac pacemaker, e.g., unipolar and bipolar sensing. Chest X-ray is obtained to evaluate for the presence of a large capnothorax from the insufflated carbon dioxide that may have tracked from the abdominal cavity through the diaphragm. A small capnothorax resolves spontaneously while a larger one may need to be aspirated.
The implanted intramuscular diaphragm electrodes are connected to a four-channel external stimulator at a percutaneous exit site. The stimulator provides a capacitively coupled charge-balanced biphasic stimulation to each electrode with a common indifferent electrode that is placed subcutaneously. The stimulator controls the charge delivered through clinician-programmed parameters of pulse amplitude, pulse duration, pulse frequency, pulse ramp, inspiration time, and respiratory rate. The clinician uses a clinical station to characterize electrode response to stimulation and program the external stimulator with the patient-specific parameters. The user simply connects the device and turns it on for use; no other controls are available or necessary for operation. For spinal-cord-injured patients the DPS was programmed to provide a stimulus that would provide a tidal volume of 15% over basal needs and for ALS patients the highest setting within safe parameters that cause no discomfort. The settings would always be below 25 mA for amplitude, frequency below 20 Hz, and pulse width below 200 μs.
ALS patients begin conditioning their diaphragm by pacing five 30-min sessions a day. If the patient is having daytime or nighttime episodes of hypoventilation, identified through either night polysomnography or hypercarbia on arterial blood gas analysis, the amount of time of DPS use can be increased and then used at night. Continuous positive airway pressure (CPAP) or noninvasive positive-pressure ventilation (NIPPV) may still be needed to maintain an upper airway and can be used in conjunction with DPS. A weaning program for SCI patients is then begun where the DPS is turned on and the ventilator is turned off. The patient’s tidal volume is checked initially with a Wright spirometer and then every 5 min. They are placed back on the ventilator when they feel uncomfortable or if their tidal volumes start dropping because of diaphragm fatigue. Initially patients may only tolerate 15 min of diaphragm pacing. Due to disuse atrophy and the conversion of muscle fibers to fast-fatigueable type during periods of inactivity, patients who have long-standing and significant respiratory paralysis will require conditioning of the diaphragm muscle in order to sustain ventilation. The diaphragm can recover quite rapidly from training so that patients and their caregiver can repeat a session every hour. The length of time it takes to go greater than four continuous hours depends on the amount of time the patient and caregivers devote to this process.
Results
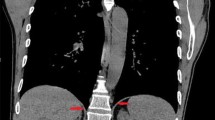
A total of 88 patients were implanted from March of 2000 until September of 2007 with 43 patients just in the first 9 months of 2007 alone. In 87 patients the diaphragm motor point was identified with subsequent implantation of the electrodes. The only failure was in the second SCI patient, who was brought to surgery and was implanted, but who was never able to have stimulated tidal volume. At surgery both diaphragms visually appeared denervated and on retrospective review of his phrenic nerve study, he was found to have a false-positive original test result. There were no diaphragm injuries, solid-organ injuries, bleeding, bowel injuries, conversion to open operations or lung injuries leading to pneumothoraxes. There were no electrode erosions to structures in the abdomen, no electrode migrations, and no late change in electrode impedance. No diaphragm electrodes have broken to date. There was one delayed suture granuloma causing an infection at the epigastric port site where the diaphragm electrodes were connected to separate electrodes that were subsequently tunneled through the skin. This was treated by externalizing the electrodes and the DPS system was still functional. The second set of connectors is no longer used as the electrodes implanted in the diaphragm are now the same ones tunneled to the exit site. There were two superficial wound infections along the tunneled wires, one in each group. In the SCI patient a chronic gastro-cutaneous fistula was closed during the DPS implantation and in the ALS patient a simultaneous gastrostomy was done, so both cases had contamination. With oral antibiotics and shortening and retermination of the tunneled electrodes, both infections resolved. No electrodes had to be removed or stopped functioning. Every patient as part of the study had intraoperative chest X-ray and in 21 out of 50 SCI patients (42%) air was observed above the diaphragm. This was only observed in five of the ALS patients (13%). This was classified as a capnothorax secondary to air tracking above the diaphragm. It was treated with either observation or simple aspiration. The capnothoraxes caused no hemodynamic or respiratory problems. Table 1 compares the tracked adverse events between the two groups of implanted patients.
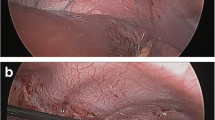
A significant difference was noted in the ALS diaphragms. Figure 3 shows the appearance of the partially denervated diaphragm, showing radial bands of pale denervated muscle. The ALS diaphragms had weaker contractions with the typical mapping stimulation. At times no quantitative change in abdominal pressure was sensed and no qualitative observation of diaphragm movement was seen. In these cases burst stimulation was then used, which involved using a train of stimulation of 1 s (Fig. 4). This would cause diaphragm movement and confirm the optimum motor point for implantation. If the diaphragm was significantly weaker the abdominal insufflation pressure would be lowered to allow visualization of diaphragm movement. Using these additional techniques allowed appropriate implantation of DPS electrodes in all of the ALS patients. Postoperative management was also different between the two groups of patients. SCI patients were easily returned to their ventilators postoperatively with no respiratory events. The ALS patients had significant respiratory compromise preoperatively yet were not on ventilators preoperatively. One of the safety aspects that was tracked during the trial was the ability to safely extubate these patients postoperatively. All patients who were on noninvasive positive-pressure ventilation (NIPPV) preoperatively were immediately placed back on NIPPV in the recovery room. Also short-acting narcotics and amnestics were used during the procedure to decrease the chance for respiratory drive suppression at the case conclusion. It was identified early in the study that ALS patients were being extubated more easily than expected. This was studied in ten of the subjects by measuring the respiratory system compliance at the end of the procedure. DPS was synchronized with the anesthesiology ventilator and the change of respiratory compliance was measured before and after the use of DPS [15]. There was a 19% increase of respiratory compliance when DPS was used. DPS was done routinely at the end of the procedure to help with extubation.
Twitch versus burst stimulation patterns. Mapping diaphragms involves a single twitch stimulation with quantitative measurement of abdominal pressures and qualitative assessment of diaphragm contraction. In a partially denervated diaphragm a twitch will not give a consistent abdominal pressure so a burst of 1 s (usually used when actually breathing for a patient) will allow a qualitative assessment of diaphragm contraction
The SCI patients were implanted with DPS an average of 5.6 years from their injury. The shortest time from injury was 3 months and the longest time was 27 years from injury while being on mechanical ventilation the entire time. Age at implant ranged from 18 to 74 years old with the average age being 36 years. Ten of the patients were injured as children and this subgroup has been reported [16]. There were 37 males and 13 females. The predominant source of injury was motor vehicle accidents (40%), followed by sports injuries (40%) and all others (20%). There has been over 97 years of active implant time with average follow-up of 2.0 ± 1.5 years (median 1.6 years, range 0.5–8.0 years). After conditioning of the diaphragm 98% of the patients were able to produce tidal volume with DPS of 15% over their basal requirements. Ninety-six percent of the patients presently use DPS for greater than four continuous hours. Over 50% of the patients had utilized DPS for over 24 continuous hours. There are a total of 44 patients that are actively using the device. Five patients have died from causes unrelated to the device, all of whom had achieved the success criteria of tidal volumes greater than basal requirements and more than 4 h of continuous use. One patient was never able to pace.
Ten SCI patients had pre-existing cardiac pacemakers and all underwent successful implantation. All pacemakers were interrogated both intraoperatively and postoperatively at nominal and most sensitive sensing settings for the cardiac pacemaker and in sensing modes supported by the cardiac pacemaker, e.g., unipolar and bipolar sensing. There were no device-to-device interactions with the DPS system when appropriately programmed.
The initial pilot group of 16 ALS patients was implanted between March of 2005 and March of 2007. Average age was 50 ± 10 years, and 13 of them were male. The FVC at implantation was 59 ± 13%. They presented on average 23 months post diagnosis of ALS. The patients were safely implanted with simultaneous feeding tubes being placed. Postoperative fluoroscopic visualization of diaphragm movement showed DPS can improve diaphragm excursion with diaphragm stimulation compared with the patient’s maximal voluntary effort when there is more upper motor neuron involvement of the phrenic motor neurons. After conditioning, the diaphragm was thicker when assessed with ultrasound (P-value 0.02). After conditioning the diaphragm with the DPS, preliminary results show an average rate of decline in FVC of 0.9% per month from the pre-implantation decline of 2.4% a month, which extrapolates to an additional 24 months of ventilator-free survival. Additional findings include: DPS can convert fast-twitch glycolytic (IIb) to functional slow-twitch oxidative muscle (I) fibers; DPS improves posterior lobe lung ventilation; DPS increases lung compliance, leading to decreased work of breathing; and patients have started utilizing DPS to improve nighttime ventilation. It has been found that ALS patients develop central hypoventilation that can be overcome with night DPS use.
The initial 20 patients implanted in the pivotal phase of the trial between March and September of 2007 were 55 ± 9 years old, and 70% were male. They had a FVC of 60 ± 12% predicted and were 22 ± 20 months post diagnosis at implant. They all tolerated surgery and their results are continuing to be followed as part of a 100-patient multicenter trial.
In the two compassionate-use patients FVC was 20 and 30% predicted and they both only had a small amount of diaphragm movement visualized under fluoroscopy. As part of this study their diaphragm EMG was evaluated post conditioning and showed an increase in size that correlated to improved movement of the diaphragm.
Discussion
The minimally invasive surgical technique of placing electrodes into the diaphragm can be safely performed at multiple independent hospital sites for the indications of ventilator-dependent tetraplegia and ALS. The only failure for implantation was in a patient who did not have an intact phrenic nerve. One of the key basic concepts of intramuscular electrodes is that, once all of the motor neurons controlling a muscle die or a nerve is completely severed, electrical stimulation at the motor point cannot cause a muscle contraction. This concept is why we identified the major differences in implantation between ALS and SCI: although ALS patients were breathing on their own, their diaphragms were much weaker during testing for the motor point. Their diaphragms were partially denervated and therefore it was more difficult to identify the motor point requiring the additional test of burst stimulation to confirm the correct implantation site.
There are no previous publications concerning general anesthesia or laparoscopy in ALS patients and the results in these 38 patients have shown that, with appropriate postoperative management, surgery can be safely performed. The 1-month mortality rate for a gastrotomy tube alone has been reported to be as high as 25% [17]. There were no perioperative mortalities in this report in any of the 38 patients, with ten undergoing simultaneous gastrostomies. The patients tolerated the procedures well given that 11 of the patients had FVC below 50% predicted and eight patients had already begun to retain CO2 because of respiratory problems. This can partially be explained by stimulation of the diaphragm during surgery, which leads to decreased posterior lobe atelectasis. The increase in compliance that we see with DPS also leads to decreased work of breathing, which is beneficial to this group of patients.
A recent review of surgery in patients with spinal cord injury can also be compared to diaphragm pacing surgery. The large Department of Veterans Affairs (VA) computer dataset was analyzed for spinal-cord-injured patients who underwent surgery (ranging from aneurysm repair to appendectomy), revealing operative mortality rates ranging from 2 to 7% [18]. There have been no perioperative deaths in the DPS trials. The reported complication rate in the VA dataset ranged from 23% (for appendectomy) to 57% (for aneurysm repair). In the DPS trials there have been none of the commonly tracked perioperative complications, including venous thrombosis, pulmonary embolus, and pulmonary infections.
Another comparison group could be patients with gastric pacemaker since this also involves a transabdominal stimulator, although the pulse generator is implanted in these subjects. In one large trial of 55 patients implanted there was one immediate perioperative death, while there were none to date in the DPS trials [19]. In this report three devices and wires had to be surgically removed for infection while no DPS wires have had to be surgically removed for infections. In addition three patients had surgical revision of the gastric pacemaker while only our first patient in the SCI trial had to have additional wires placed for success. Since this initial change in technique no patients have required revisional surgery [12].
There have been no lung injuries, but air has been identified above the diaphragm due to CO2 tracking from the pressurized abdomen to the pleural space. This has been described as a capnothorax and was prospectively tracked. In no case has the gas in the pleural space been from an injury to the lung itself. The latter would have been identified from having a continuing air leak from a chest tube or an increasing size pneumothorax after the laparoscopic procedure. All of the patients, by the nature of their tetraplegia, are on positive-pressure ventilation during and immediately after anesthesia, which would have potentially increased any air leaking from an iatrogenic lung injury. Capnothorax is therefore regarded differently from a pneumothorax related to lung injury.
During the course of this study, the phenomenon of gas tracking into the pleural space was also being described in other laparoscopic surgeries. Clements and colleagues [20] at Vanderbilt did chest X-rays on 45 patients undergoing laparoscopic foregut surgery that involved operations around the diaphragm and 21 (47%) had extra-abdominal gas identified. In another report extra-abdominal gas was identified in 85% of patients undergoing laparoscopic Nissen fundoplication when a more sensitive test, such as computed tomography (CT) scan, was performed after surgery [21]. Clements states that this air caused no clinically significant events that altered the course of the patients, and we found similar results in our tetraplegics undergoing diaphragm pacing, although a slightly different algorithm is used based on these patients. Patients undergoing laparoscopic foregut have both a normal inspiratory and expiratory system so that CO2 that has tracked to the chest space is rapidly absorbed. The lung can expand rapidly when the patients have a large inspiratory force during awakening from anesthesia and the collapsed airways can expand. Tetraplegics implanted with the DPS have no inspiratory or expiratory capabilities and are on set-volume ventilators. Their lungs therefore do not typically expand to fill this empty space. In the first patient in which this was seen, we placed a chest tube to re-expand the lung. This case prompted a change in the management of subsequent patients with capnothorax. We found that capnothorax may, in most cases, be observed and allowed to resolve expectantly in nontetraplegic patients. Due to the rare nature of ventilator-dependent tetraplegia and the fact that many patients utilized air travel to go to the implantation site, we did not want patients traveling with a nonsymptomatic but stable capnothorax. The present management of an observed capnothorax is to treat by initially giving much larger mechanical breaths via the patients’ tracheostomy and repeating the chest X-ray in the recovery room. If the capnothorax is still present, then it is aspirated by a method most familiar to the implanting surgeon (either a small pediatric chest tube or a thoracentesis needle).
Recent articles have shown that mechanical ventilation negatively affects the diaphragm, with over 50% atrophy and conversion to the fast-twitch type II muscles in less than 1 day [22]. With the large body of experience with the DPS system maintaining muscle mass and converting the diaphragm into functional type I muscle fibers, the future of diaphragm pacing should include much earlier implantation in SCI patients and use in intensive care unit (ICU) to help patients be removed from mechanical ventilation. Animal studies have led to less invasive methods of application including the possibility of natural orifice transluminal endoscopic surgery (NOTES) techniques so that the pacing wires could be placed at the bedside for patients in the ICU at the time of their percutaneous endoscopic gastrostomy (PEG) tube placement [23].
Conclusion
DPS can be safely implanted in these two patient groups. It should be offered to all SCI patients on ventilator and earlier after their injury. The ALS results have shown in the trials to date that, by maintaining diaphragm strength, patients have delayed their need for a ventilator by up to 24 months.
References
Devivo MJ, Go BK, Jackson AB (2002) Overview of the national spinal cord injury statistical center database. J Spinal Cord Med 25:335–338
Ragnarsson KT (2008) Functional electrical stimulation after spinal cord injury: current us, therapeutic effects and future directions. Spinal Cord 46:255–274
Peterson DK, Nochomovitz ML, DiMarco AF, Mortimer JT (1986) Intramuscular electrical activation of the phrenic nerve. IEEE Trans Biomed Eng 33:342–351
Nochomovitz ML, Peterson DK, Stellato TA (1988) Electrical activation of the diaphragm. Clin Chest Med 9:349–358
Peterson DK, Nochomovitz ML, Stellato TA, Mortimer JT (1994) Long-term intramuscular electrical activation of phrenic nerve: efficacy as a ventilatory prosthesis. IEEE Trans Biomed Eng 41:1127–1135
Schmit BD, Stellato TA, Miller ME, Mortimer JT (1998) Laparoscopic placement of electrodes for diaphragm pacing using stimulation to locate the phrenic nerve motor points. IEEE Trans Rehabil Eng 6:382–390
Aiyar H, Stellato TA, Onders RP, Mortimer JT (1999) Laparoscopic implant instrument for the placement of intramuscular electrodes in the diaphragm. IEEE Trans Rehabil Eng 7:360–371
Onders RP, Aiyar H, Mortimer JT (2004) Characterization of the human diaphragm muscle with respect to the phrenic nerve motor points for diaphragmatic pacing. Am Surg 70:241–247
Dimarco AF, Onders RP, Kowalski KE, Miller ME, Ferek S, Mortimer JT (2002) Phrenic nerve pacing in a tetraplegic patient via intramuscular diaphragm electrodes. Am J Respirat Crit Care Med 166:1604–1606
DiMarco AF, Onders RP, Ignangi AI, Kowalski KE, Stephan S, Mortimer JT (2005) Phrenic nerve pacing via intramuscular diaphragm electrodes in tetraplegic subjects. Chest 127:671–677
Onders RP, Ignagni AI, Aiyer H, Mortimer JT (2004) Mapping the phrenic nerve motor point: the key to a successful laparoscopic diaphragm pacing system in the first human series. Surgery 136:819–826
Onders RP, Ignagni AI, DiMarco AF, Mortimer JT (2005) The learning curve of investigational surgery: lessons learned from the first series of laparoscopic diaphragm pacing for chronic ventilator dependence. Surg Endosc 19:633–637
Dimarco AF, Onders RP, Ignagni A, Kowolski KE (2006) Inspiratory muscle pacing in spinal cord injury: case report and clinical commentary. J Spinal Cord Med 29:95–108
Onders R, Katirji B, Schilz R, Elmo M, Sivashankaran S, Ignagni A (2007) Motor point diaphragm pacing in patients with MND/ALS: long term follow-up of completed safety and feasibility study. Amyotroph Lateral Scler 5(S):82
Onders R, Schilz R, Sivashankara S, Karirji B, Elmo M, Ignagni A (2007) Diaphragm pacing as a short-term assist to positive pressure mechanical ventilation in critical care patients. Chest 132:571S
Onders RP, Elmo MJ, Ignagni AR (2007) Diaphragm pacing stimulation system for tetraplegia in individuals injured during childhood or adolescence. J Spinal Cord Med 30:25–29
Forbes RR, Colville S, Swingler RJ (2004) Frequency, timing and outcome of gastrostomy tubes for amyotrophic lateral sclerosis/motor neuron disease. J Neurol 251:813–817
Johnson FE, Virgo KS (2007) Major surgery in patients with previous spinal cord injury. J Spinal Cord Med 30:408–409
Forster J, Sarosiek I, Lin Z, Durham S, Denton S, Roeser K, McCallum RW (2003) Further experience with gastric stimulation to treat drug refractory gastroparesis. Am J Surg 186:690–695
Clements RH, Reddy S, Holzman MD, Sharp KW, Olsen D, Holcomb GW, Richards WO (2000) Incidence and significance of pneumomediastinum after laparoscopic esophageal surgery. Surg Endosc 14:553–555
Harris JA, Gallo CD, Brummett DM, Mullins MD, Figueroa-Ortiz RE (2001) Extra-abdominal pneumodissection after laparoscopic antireflux surgery. Am Surg 67:885–889
Levine S, Nguyen T, Talyor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrager J (2009) Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. NEJM 358:1327–1335
Onders R, McGee M, Marks J, Chak A, Schilz R, Rosen M, Ignagni A, Faulx A, Elmo MJ, Schmoisch S, Ponsky J (2007) Diaphragm pacing with natural orifice tranluminal endoscopic surgery (NOTES): potential for difficult to wean intensive care unit (ICU) patients. Surg Endosc 21:475–479
Conflicts of Interest Disclosure
Case Western Reserve University, Dr. Raymond Onders and University Hospitals Health System have intellectual property rights involved with the diaphragm pacing system and equity in Synapse Biomedical who manufactures the device.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Onders, R.P., Elmo, M., Khansarinia, S. et al. Complete worldwide operative experience in laparoscopic diaphragm pacing: results and differences in spinal cord injured patients and amyotrophic lateral sclerosis patients. Surg Endosc 23, 1433–1440 (2009). https://doi.org/10.1007/s00464-008-0223-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-008-0223-3