-
PDF
- Split View
-
Views
-
Cite
Cite
Nens van Alfen, Baziel G. M. van Engelen, The clinical spectrum of neuralgic amyotrophy in 246 cases, Brain, Volume 129, Issue 2, February 2006, Pages 438–450, https://doi.org/10.1093/brain/awh722
Close - Share Icon Share
Abstract
We investigated the symptoms, course and prognosis of neuralgic amyotrophy (NA) in a large group of patients with idiopathic neuralgic amyotrophy (INA, n = 199) and hereditary neuralgic amyotrophy (HNA, n = 47) to gain more insight into the broad clinical spectrum of the disorder. Several findings from earlier smaller-scale studies were tested, and for the first time the potential differences between the hereditary and idiopathic phenotypes and between males and females were explored. Generally, the course of the pain manifests itself in three consecutive phases with an initial severe, continuous pain lasting for ∼4 weeks on average. Sensory involvement was quite common and found in 78.4% of patients but was clinically less impairing than the initial pain and subsequent paresis. As a typically patchy disorder NA can affect almost any nerve in the brachial plexus, although damage in the upper and middle trunk distribution with involvement of the long thoracic and/or suprascapular nerve occurred most frequently (71.1%). We found no correlation between the distribution of motor and sensory symptoms. In INA recurrent attacks were found in 26.1% of the patients during an average 6 year follow-up. HNA patients had an earlier onset (28.4 versus 41.3 years), more attacks (mean 3.5 versus 1.5) and more frequent involvement of nerves outside the brachial plexus (55.8 versus 17.3%) than INA patients, and a more severe maximum paresis, with a subsequent poorer functional outcome. In males the initial pain tended to last longer than it did in females (45 versus 23 days). In females the middle or lower parts of the brachial plexus were involved more frequently (23.1 versus 10.5% in males), and their functional outcome was worse. Overall recovery was less favourable than usually assumed, with persisting pain and paresis in approximately two-thirds of the patients who were followed for 3 years or more.
Introduction
Neuralgic amyotrophy (NA), which was first described as a distinct clinical syndrome in the late 19th century, is characterized by attacks of neuropathic pain and subsequent patchy paresis in the upper extremities. Apart from other names, the Parsonage–Turner syndrome is probably the second most common denomination for the disorder (Wilbourn, 1993). NA can occur as a sporadic disorder but also exists as an autosomal dominant hereditary trait, known as hereditary neuralgic amyotrophy (HNA), which predisposes to recurrent attacks of peripheral nerve damage. Very recently a mutation in the SEPT9 gene was found in several HNA families (Kuhlenbaumer et al., 2005). The attacks are currently assumed to be autoimmune in origin, but the precise mechanism is unknown (Suarez, 2005). Idiopathic NA (INA) has a reported incidence of 2–3/100 000/year (Beghi et al., 1985; MacDonald et al., 2000).
It is likely that, rather than a single disease entity, NA is a syndrome comprising different underlying mechanisms, phenotypes and prognoses (Byrne, 1987; England, 1987; Cruz-Martinez et al., 2002). The present study aimed to gain a deeper insight into the various clinical manifestations of NA and to further delineate its course and prognosis, thus facilitating a better distinction from other disorders. Since 2000, our institute has been the national referral centre for NA patients in the Netherlands. The ultimate goal of our explorations is to help clinicians identify patients more rapidly—making early therapeutic intervention possible—to find clues to help target pathophysiological research better, and finally, to provide patients and caregivers with a more detailed overview of the condition, its prognosis and the most effective therapeutic approaches.
Methods
Patients
To be included in our consecutive case-series analysis patients needed to have suffered an attack of acute, painful, patchy brachial and/or lumbosacral plexopathy that followed a monophasic course, for which no specific (other) aetiology could be identified at onset and after follow-up. Our selection criteria followed the diagnostic guidelines as put forward in a 1999 European Neuromuscular Centre workshop for HNA, but, obviously, regardless of family history (Kuhlenbaumer et al., 2000). Because NA is known to involve a wide phenotypic variation (e.g. Byrne, 1987; England, 1999), we also included patients in whom paresis preceded pain, or who suffered painless but otherwise typical attacks, or attacks that also involved nerves outside the brachial plexus. Although we mainly tried to include patients seen at our centre in a prospective manner, data was also collected retrospectively, and well-documented cases observed by our colleagues elsewhere were also included. Episodes with symptoms in these patients were only counted as attacks if they had been confirmed by clinical assessment.
Statistics
The demographic, epidemiological and clinical data available from chart reviews were recorded as variables in an SPSS database. Statistical analyses were performed using SPSS version 12 (SPSS Inc, Chicago, IL, USA). For each variable the number of available cases was noted, and percentages represent parts of the available cases per variable unless noted otherwise. In each patient, one attack was further analysed for time course, pain characteristics, localization and severity of paresis, sensory symptoms, and functional impairments experienced. We also collected information on the initial diagnosis and the time elapsed before the diagnosis of NA was established. Some of the continuous variables were redefined in several discrete categories to allow further calculations (see below).
Clinical data
Pain was assessed using the Numerical Rating Scale (NRS; range 0–10). The maximum severity of the paresis during an attack was defined as the average MRC grade of the affected muscles and denoted as mild (MRC 4–5−), moderate (MRC 3–4−) or severe (MRC <3). During processing of the data, the loci of the pain as described by the patients were clustered into four different patterns according to the most proximal locus involved. The distribution of sensory symptoms was first clustered in five anatomical regions, and again according to their truncal distribution in the plexus for comparison with motor deficits. Besides an upper, middle and lower trunk, a fourth region, involving the more proximal parts of the plexus or cervical roots, was encoded. Likewise, we also classified the distribution of the paresis into six anatomical regions, and again according to truncal distribution within the plexus. In the anatomical regions, attacks in the upper and/or middle part of the plexus in which the long thoracic nerve was affected were classified separately, because the presence of a winged scapula seems to facilitate clinicians in recognizing NA. We did not systematically assess the presence of minor dysmorphisms in HNA patients or the prevailing clinical course (intermittent or chronic-undulating) in the HNA families.
The results of ancillary investigations, e.g. blood analyses, CSF studies, electrodiagnostic examinations and imaging studies, were noted when available. In a number of patients who had had a painless attack or a concomitant pressure palsy (e.g. carpal tunnel syndrome), or a family history of pressure palsies, DNA was tested for the presence of a PMP22 deletion found in hereditary neuropathy with liability to pressure palsies (HNPP).
We evaluated various treatments and their efficacy. Pain relief resulting from medication was subjectively categorized by the patient as ‘good’, ‘some’ or ‘none’.
Because there is no standard definition of recovery for NA, several parameters were recorded representing residual symptoms (neuropathic and musculoskeletal pain; paresis), a subjective estimate of overall recovery (patient judges ‘full recovery’ based on a yes/no question; overall recovery percentages were estimated in six categories: none, <50, 50–70, 70–80, 80–90, 90–100%) and functional outcome (i.e. the ability to work; modified Rankin score). We excluded 43 patients from this analysis because they had received treatment with prednisone for one or more of their attacks; they will be reported on separately. Because of the variable follow-up times within our case series, we grouped the patients in five categories delineated by the duration of the follow-up since the last NA attack: 0–6 months, 6–12 months, 1–2 years, 2–3 years and 3 years or more. For comparison with other studies, the long-term results for patients in the latter category are presented in more detail. Due to the considerable between-patients variation in follow-up durations and intervals, we were unable to perform a survival analysis for recovery over time for the group as a whole.
Subgroup analysis
On clinical grounds we expected there would be no significant difference in clinical characteristics between INA and HNA patients, except for the age of onset and the total number of attacks, and possibly for the involvement of nerves outside the distribution of the brachial plexus, such as the phrenic, lumbosacral plexus, intercostals or cranial nerves, which has mainly been reported in HNA (van Alfen et al., 2000). We also compared males and females to see whether there are sex-related differences in NA. Preceding further analysis, differences for both subgroups were tested for categorical variables using the Pearson's χ2 method (two-sided testing for a significance level of ≤0.05); an independent Student's t-test was used for continuous variables. When different, results for these variables were reported separately for INA and HNA patients or males and females; all other variables were reported for the patient group as a whole.
Descriptive data on patients who had received open-label prednisone treatment are provided, and where possible compared with untreated patients with respect to epidemiological and outcome variables. Differences for both subgroup divisions were also tested with Pearson's χ2 and independent Student's t-tests. Two patients were excluded from the analysis because they had participated in a placebo-controlled trial with prednisone.
We used linear regression to evaluate attack and recovery over time in a subgroup of patients who had suffered only one attack and had not received prednisone. Dependent variables were NRS scores at onset and follow-up, severity of the paresis during the attack, maximum strength level at recovery, estimated recovery percentage and Rankin score. Independent variables were sex, age, HNA, presence of an antecedent event, contractions at wrists or fingers, presence of distal vasomotor dysfunction, weight loss and time (five categories, see above).
Results
Study population characteristics
A total of 246 patients with either INA or HNA were identified from our centres' neuromuscular database, of whom 104 (42.3%) were evaluated by the first author (N.A.). All but 14 patients were evaluated at our centre (94.3%); 192 (78.1%) could also be followed prospectively, and retrospective data only was available in 54 patients (21.9%). We included 174 (70.7%) patients that met the ENMC criteria, and 72 (29.3%) with a phenotypic variation, including 9 (3.7%) patients with painless attacks, 8 (3.3%) with paresis preceding pain and 55 (22.4%) who also had involvement of nerves outside the brachial plexus, either in the evaluated (27, 11%) or in a previous attack (28, 11.4%). The study population consisted of 67.5% males (68.3% in INA and 63.8% in HNA, difference not statistically significant); 87% of the patients were right-handed, 11.1% left-handed and 1.9% ambidextrous, and 19% had a family history of the disorder (HNA, n = 47, from 36 families), which had been confirmed in 35 patients by examination of an affected family member. The data from seven of the HNA patients were also used in a previous study (van Alfen et al., 2000); the total number of HNA patients evaluated in our centre at the time of the preparation of the manuscript was 52.
Of the 34 clinical variables 6 differed significantly between HNA and INA patients: age of onset (P < 0.0001), total number of attacks (P < 0.0001), the involvement of nerves outside the brachial plexus (P < 0.0001), increasing pain in the first few weeks after the onset of an attack (P = 0.03), maximum severity of paresis during an attack (including or excluding patients receiving prednisone treatment; P = 0.04 and 0.01, respectively), Rankin score (P = 0.008) and the legally established disability to work (‘sick leave’; P = 0.005). Males and females also differed significantly in six variables: the duration of the primary pain (P = 0.006), the presence of neuropathic pain on the last follow-up (P = 0.04), presence of atrophy (P = 0.02), pattern of motor paresis (P = 0.01), increased mechanical sensitivity of the plexus (P = 0.02) and Rankin score on follow-up (P = 0.004). The corresponding numbers and percentages for these differences are described in the specific results sections below.
The mean age of onset in INA was 41.3 years (median 40.5, range 10–79.8 years), and had a normal distribution. From the total of 194 INA patients in this category, 3.1% had experienced their first attack in childhood, i.e. before the age of 16 years. As expected, onset for HNA patients (n = 44) was earlier at a mean of 28.4 years (median 28.0, range 3–56.3 years), with 22.7% of patients suffering their first attack during childhood. After an average follow-up from onset of 6.2 years, the mean number of attacks in INA patients was 1.5 (median 1) and in HNA patients was 3.5 (median 3). The mean time before NA was diagnosed was 43.8 weeks (median 10.5) with 75% of the patients being diagnosed within 28 weeks from onset. In 61.9% of the cases patients had received a different initial diagnosis, usually either glenohumeral joint pathology or cervical radiculopathy.
Characteristics of NA attacks
General features
In 90% of the patients the onset of an NA attack was announced by the disorder's typical pain (for more details see Pain characteristics section), which usually emerged within a few hours. Even when absent initially, 62.5% of the patients still developed the pain during the course of the attack. In 60.9% of the cases the attacks began at night (between 00.00 and 07.00 h). In 71.5% they involved only one arm, and in 28.5% both arms, typically in an asymmetric fashion (97.1%). There was only one documented attack (0.4%) in an HNA patient that was not associated with paresis (pain only or ‘abortive attack’).
In 55.8% of the HNA patients, nerves outside the brachial plexus were also involved during an attack, e.g. the lumbosacral plexus (32.6%), phrenic nerve (14%), recurrent laryngeal nerve (18.6%) or other (7%; percentages do not add up due to combinations). In INA patients this type of involvement was seen less frequently (17.3%; 8.2%, lumbosacral plexus; 6.6%, phrenic nerve; 2%, recurrent laryngeal nerve; 2.6%, other).
As expected, attacks recurred in a large number (74.5%) of HNA patients. Nineteen per cent had suffered only one attack during our follow-up, 29.8% two attacks, 17% three, 10.6% four and 23.4% five or more attacks, with a maximum of 13. More surprisingly perhaps, recurrences were also seen in 26.1% of the INA patients (19.1% two, 4.5% three, 4% four and 1% five or more attacks). The median time to a recurrence slightly exceeded 2 years (109 weeks; mean 250 weeks) in INA patients and almost 6 years (300 weeks; mean 349 weeks) in HNA, but this difference was not statistically significant (P = 0.08). Two-thirds (n = 42) of the INA patients with recurrences were referred to our centre after their last attack. One-third (n = 21), out of a total of 163 INA patients (i.e. 12%) whom we prospectively followed after their first attack, suffered a recurrence during follow-up at our centre, within a median time of 6 months (mean 1.1 years) after their first visit. INA patients with recurrences were significantly younger at onset (35.0 years), compared with INA patients who had suffered only one attack (43.9 years; P < 0.001), but did not differ in other aspects. Recurrences could affect the same peripheral nerve territory in each attack (23.5%), completely different territories (32.1%), or sometimes the same and sometimes different territories (44.4%).
Further characteristics of the NA attacks are given in Table 1.
Characteristics of NA attacks
. | Percentage . | . | . | . | Available cases . | |||
|---|---|---|---|---|---|---|---|---|
| First symptom | Pain 90% | Paresis 5.8% | Sensory 2.9% | Atrophy 1.2% | 241 | |||
| Time of onset | Night (0–7 h) 60.9% | Daytime (7–18 h) 28.2% | Evening (18–0 h) 10.9% | 110 | ||||
| Localization upper extremities | One arm 71.5% | Both arms 28.5% | 242 | |||||
| Right 47.1% | Asymmetric 27.7% | |||||||
| Left 24.4% | Symmetric 0.8% | |||||||
| Hand dominance, in right-sided attacks | Right 89.1% | Left 9.9% | Ambidextrous 1% | 101 | ||||
| Hand dominance, in left-sided attacks | Right 78% | Left 17% | Ambidextrous 4.3% | 47 | ||||
. | Percentage . | . | . | . | Available cases . | |||
|---|---|---|---|---|---|---|---|---|
| First symptom | Pain 90% | Paresis 5.8% | Sensory 2.9% | Atrophy 1.2% | 241 | |||
| Time of onset | Night (0–7 h) 60.9% | Daytime (7–18 h) 28.2% | Evening (18–0 h) 10.9% | 110 | ||||
| Localization upper extremities | One arm 71.5% | Both arms 28.5% | 242 | |||||
| Right 47.1% | Asymmetric 27.7% | |||||||
| Left 24.4% | Symmetric 0.8% | |||||||
| Hand dominance, in right-sided attacks | Right 89.1% | Left 9.9% | Ambidextrous 1% | 101 | ||||
| Hand dominance, in left-sided attacks | Right 78% | Left 17% | Ambidextrous 4.3% | 47 | ||||
Characteristics of NA attacks
. | Percentage . | . | . | . | Available cases . | |||
|---|---|---|---|---|---|---|---|---|
| First symptom | Pain 90% | Paresis 5.8% | Sensory 2.9% | Atrophy 1.2% | 241 | |||
| Time of onset | Night (0–7 h) 60.9% | Daytime (7–18 h) 28.2% | Evening (18–0 h) 10.9% | 110 | ||||
| Localization upper extremities | One arm 71.5% | Both arms 28.5% | 242 | |||||
| Right 47.1% | Asymmetric 27.7% | |||||||
| Left 24.4% | Symmetric 0.8% | |||||||
| Hand dominance, in right-sided attacks | Right 89.1% | Left 9.9% | Ambidextrous 1% | 101 | ||||
| Hand dominance, in left-sided attacks | Right 78% | Left 17% | Ambidextrous 4.3% | 47 | ||||
. | Percentage . | . | . | . | Available cases . | |||
|---|---|---|---|---|---|---|---|---|
| First symptom | Pain 90% | Paresis 5.8% | Sensory 2.9% | Atrophy 1.2% | 241 | |||
| Time of onset | Night (0–7 h) 60.9% | Daytime (7–18 h) 28.2% | Evening (18–0 h) 10.9% | 110 | ||||
| Localization upper extremities | One arm 71.5% | Both arms 28.5% | 242 | |||||
| Right 47.1% | Asymmetric 27.7% | |||||||
| Left 24.4% | Symmetric 0.8% | |||||||
| Hand dominance, in right-sided attacks | Right 89.1% | Left 9.9% | Ambidextrous 1% | 101 | ||||
| Hand dominance, in left-sided attacks | Right 78% | Left 17% | Ambidextrous 4.3% | 47 | ||||
Pain characteristics
Overall, 96.3% of the patients experienced the typical, very severe, relentless, neuropathic ‘NA pain’ during their attacks. This initial pain was continuous in 93.8% patients and had a median NRS rating of 8 at onset and 9 at maximum intensity. Put differently, initially 60% and subsequently 90% of patients had an NRS score of 7 or more. In the subgroup of 133 patients who had only suffered one untreated attack, the NRS score at onset was on average two points higher in HNA patients (7.3 versus 9.4, P = 0.04).
Pain had a stuttering onset in 18.7%, which implies that pain could have been present on-and-off for a few weeks before the ‘real’ attack, characterized by the continuous severe pain, began. After onset, pain severity increased even further during the first few weeks in 38.5% of the INA patients versus 18% of the HNA patients. In 88.6% of all patients the pain was worse at night and caused sleep disturbances in 93.5%. There was evidence of increased mechanical sensitivity (e.g. pain elicited by movement of or pressure on the affected limb, a ‘Lasegue sign of the arm’) in 80% of the males and in 97.4% of the females.
In males the initial pain lasted on average twice as long (45.3 days, median 21 days) as it did in females (23.3 days, median 17 days). For the group as a whole the average pain duration was 27.5 days (median 19.5 days). In 4.9% of the cases the pain disappeared within 24 h, in 22.7% it persisted between 1 and 7 days and in 10% it lasted 60 days or more. Six patients who reported their initial pain to have lasted for over a year were excluded as outliers. It is likely that, as time progressed, they suffered from one of the subsequent pain types reported in NA (see below) but had been unable to distinguish between the pain types.
Many patients (76.5%) described two additional pain phases in the course of their attack. The continuous initial pain was followed by severe neuropathic stabbing or shooting pains usually elicited by movement, lying on or prolonged posturing of the affected limb, which lasted from a few weeks to several months (data not quantified) and dissipated gradually. Many patients (65.1%) reported a subsequent persisting musculoskeletal-type pain, usually localized to the origin or insertion of the paretic—or compensating—muscles, especially in the periscapular, cervical and occipital regions. A total of 23% of the males and 37.5% of the females suffered from neuropathic pains even at the final follow-up. In addition, sometimes patients also developed pain related to glenohumeral joint pathology after an NA attack with paresis of the rotator cuff muscles or decreased glenohumeral excursions due to paresis, and 17% developed a true frozen shoulder (glenohumeral adhesive capsulitis) during the course of their attack. Glenohumeral subluxation or luxation occurred in 8.4% of cases, and 9.6% developed contractures of the wrist or fingers due to long-standing distal paresis. On follow-up, 29% developed chronic, usually therapy-resistant, continuous pains in the previously affected region.
The locus of the initial pain was quite variable: the patients described 32 different patterns. After re-clustering the loci into four regions, which were either uni- or bilateral, in 39.7% the pain was found to occur in and/or radiated from the shoulder to the arm, in 35.4% from the cervical spine or neck down into the arm, in 18.8% from the scapular or dorsal region to the chest wall and/or arm, and in 6.1% the pain was confined to a lower plexus distribution (e.g. medial arm and/or hand, axilla). A small proportion of the patients reported pain in a very restricted area: scapula, neck or upper arm only (1.3%, each), or the posterior cervical and chest-wall region (3.5%).
In about two-thirds of the patients the initial pain could be partly relieved by avoiding movement or certain postures. The local application of warmth provided relief in 26 out of 38 patients. Many (83.5%) used analgesics during their attack. The medication used and the subjective relief it provided are listed in Table 2. The most effective pain relief was achieved with the combination of an NSAID and an opiate (e.g. diclofenac slow-release 100 mg bid with slow-release morphine 10–30 mg bid).
Analgesics used for initial pain and their effects according to the patients
| Analgesic . | Subjective effect . | . | . | Cases . | ||
|---|---|---|---|---|---|---|
. | Good (%) . | Some (%) . | None (%) . | . | ||
| Acetaminophen | 20 | 60 | 20 | 5 | ||
| NSAID | 2.2 | 43.5 | 54.3 | 46 | ||
| Opiates (including tramadol) | 31.6 | 52.6 | 15.8 | 19 | ||
| NSAID with any opiate | 60.7 | 39.3 | 0 | 28 | ||
| Any with co-analgesic* | 4.3 | 69.6 | 26.1 | 23 | ||
| Analgesic . | Subjective effect . | . | . | Cases . | ||
|---|---|---|---|---|---|---|
. | Good (%) . | Some (%) . | None (%) . | . | ||
| Acetaminophen | 20 | 60 | 20 | 5 | ||
| NSAID | 2.2 | 43.5 | 54.3 | 46 | ||
| Opiates (including tramadol) | 31.6 | 52.6 | 15.8 | 19 | ||
| NSAID with any opiate | 60.7 | 39.3 | 0 | 28 | ||
| Any with co-analgesic* | 4.3 | 69.6 | 26.1 | 23 | ||
Amitriptyline, carbamazepine or gabapentin.
Analgesics used for initial pain and their effects according to the patients
| Analgesic . | Subjective effect . | . | . | Cases . | ||
|---|---|---|---|---|---|---|
. | Good (%) . | Some (%) . | None (%) . | . | ||
| Acetaminophen | 20 | 60 | 20 | 5 | ||
| NSAID | 2.2 | 43.5 | 54.3 | 46 | ||
| Opiates (including tramadol) | 31.6 | 52.6 | 15.8 | 19 | ||
| NSAID with any opiate | 60.7 | 39.3 | 0 | 28 | ||
| Any with co-analgesic* | 4.3 | 69.6 | 26.1 | 23 | ||
| Analgesic . | Subjective effect . | . | . | Cases . | ||
|---|---|---|---|---|---|---|
. | Good (%) . | Some (%) . | None (%) . | . | ||
| Acetaminophen | 20 | 60 | 20 | 5 | ||
| NSAID | 2.2 | 43.5 | 54.3 | 46 | ||
| Opiates (including tramadol) | 31.6 | 52.6 | 15.8 | 19 | ||
| NSAID with any opiate | 60.7 | 39.3 | 0 | 28 | ||
| Any with co-analgesic* | 4.3 | 69.6 | 26.1 | 23 | ||
Amitriptyline, carbamazepine or gabapentin.
Paresis and atrophy
In the attacks characterized by initial pain, the first signs of weakness appeared within 24 h in 33.5% of the patients, after 1–7 days in 39.3% and 1–2 weeks in 14.1%. In 27.2% of all cases paresis did not manifest itself until >2 weeks later. The mean time to the onset of weakness was 13.6 days in the male and 8 days in the female patients, but the difference was not statistically significant (P = 0.18). An increment of the paresis occurred in 30.2% of the cases with severity progressing over a period of days (8.6%), weeks (16%) or months (5.6%).
The distribution of the motor deficits as found for the male and female NA patients, clustered in anatomical regions is shown in Table 3. Females more often had involvement of the middle or lower parts of the brachial plexus (23.1 versus 10.5% in males; P = 0.01). Overall, paresis of the distribution of the upper part of the brachial plexus was by far the most common (71.1%), either with (50.2%) or without (20.9%) involvement of the long thoracic nerve. Any part of the plexus, and clinically any muscle, could be involved (seeTable 4), but paresis of the infraspinate (72%) and serratus anterior muscle (70%) was the most common, and involvement of the sternocleidomastoid (7.2%) or neck extensors (1.5%) infrequent. Although the maximum severity of the paresis during an attack differed statistically between INA and HNA patients (P = 0.04), it was generally severe in both groups (64.9 and 69.8%, respectively). In these severely affected patients, paralysis (MRC grade 0/5) was more frequent in HNA than in INA (11.6 versus 3.7%). Conversely, there were more INA patients (13.1%) than HNA patients (2.3%) with a mild maximum motor deficit. The remainder of the patients (22% INA and 27.9% HNA) suffered a moderate paresis.
Distribution of motor paresis for male and female patients regrouped in anatomical regions
| Pattern . | Percentage in males . | Percentage in females . | Total cases . |
|---|---|---|---|
| (1) Classic NA: upper and/or middle plexus including long thoracic nerve | 50.9 | 50 | 121 |
| (2) Upper and/or middle plexus without long thoracic nerve | 24.8 | 12.8 | 50 |
| (3) All parts plexus affected | 13.7 | 14.1 | 37 |
| (4) Middle plexus and/or posterior cord predominant | 6.2 | 6.4 | 13 |
| (5) Lower plexus predominant | 3.1 | 7.7 | 10 |
| (6) Anterior interosseus nerve predominant | 1.2 | 9.0 | 9 |
| Pattern . | Percentage in males . | Percentage in females . | Total cases . |
|---|---|---|---|
| (1) Classic NA: upper and/or middle plexus including long thoracic nerve | 50.9 | 50 | 121 |
| (2) Upper and/or middle plexus without long thoracic nerve | 24.8 | 12.8 | 50 |
| (3) All parts plexus affected | 13.7 | 14.1 | 37 |
| (4) Middle plexus and/or posterior cord predominant | 6.2 | 6.4 | 13 |
| (5) Lower plexus predominant | 3.1 | 7.7 | 10 |
| (6) Anterior interosseus nerve predominant | 1.2 | 9.0 | 9 |
Distribution of motor paresis for male and female patients regrouped in anatomical regions
| Pattern . | Percentage in males . | Percentage in females . | Total cases . |
|---|---|---|---|
| (1) Classic NA: upper and/or middle plexus including long thoracic nerve | 50.9 | 50 | 121 |
| (2) Upper and/or middle plexus without long thoracic nerve | 24.8 | 12.8 | 50 |
| (3) All parts plexus affected | 13.7 | 14.1 | 37 |
| (4) Middle plexus and/or posterior cord predominant | 6.2 | 6.4 | 13 |
| (5) Lower plexus predominant | 3.1 | 7.7 | 10 |
| (6) Anterior interosseus nerve predominant | 1.2 | 9.0 | 9 |
| Pattern . | Percentage in males . | Percentage in females . | Total cases . |
|---|---|---|---|
| (1) Classic NA: upper and/or middle plexus including long thoracic nerve | 50.9 | 50 | 121 |
| (2) Upper and/or middle plexus without long thoracic nerve | 24.8 | 12.8 | 50 |
| (3) All parts plexus affected | 13.7 | 14.1 | 37 |
| (4) Middle plexus and/or posterior cord predominant | 6.2 | 6.4 | 13 |
| (5) Lower plexus predominant | 3.1 | 7.7 | 10 |
| (6) Anterior interosseus nerve predominant | 1.2 | 9.0 | 9 |
Muscles affected
| Muscle . | Percentage affected . | Cases examined . |
|---|---|---|
| Infraspinatus | 71.8 | 216 |
| Serratus anterior | 70 | 230 |
| Supraspinatus | 65.7 | 204 |
| Biceps brachii | 61 | 223 |
| Rhomboids | 54.2 | 179 |
| Pronator teres | 52.3 | 155 |
| Brachioradialis | 48.1 | 162 |
| Wrist extensors | 47.4 | 209 |
| Deltoid | 46 | 226 |
| Triceps brachii | 43.4 | 221 |
| Wrist flexors | 36.3 | 212 |
| Finger extensors | 36.3 | 193 |
| Pronator quadratus | 33.3 | 45 |
| Deep flexors digits I and II | 30.6 | 180 |
| Dorsal interosseus | 30 | 217 |
| Adductor pollicis | 27.4 | 84 |
| Thumb extensors | 27.2 | 151 |
| Teres major | 26.4 | 129 |
| Abductor pollicis | 26.3 | 133 |
| Trapezius | 19.9 | 221 |
| Pectoralis major | 14.8 | 209 |
| Sternocleidomastoid | 7.2 | 181 |
| Paraspinal neck extensors | 1.5 | 198 |
| Muscle . | Percentage affected . | Cases examined . |
|---|---|---|
| Infraspinatus | 71.8 | 216 |
| Serratus anterior | 70 | 230 |
| Supraspinatus | 65.7 | 204 |
| Biceps brachii | 61 | 223 |
| Rhomboids | 54.2 | 179 |
| Pronator teres | 52.3 | 155 |
| Brachioradialis | 48.1 | 162 |
| Wrist extensors | 47.4 | 209 |
| Deltoid | 46 | 226 |
| Triceps brachii | 43.4 | 221 |
| Wrist flexors | 36.3 | 212 |
| Finger extensors | 36.3 | 193 |
| Pronator quadratus | 33.3 | 45 |
| Deep flexors digits I and II | 30.6 | 180 |
| Dorsal interosseus | 30 | 217 |
| Adductor pollicis | 27.4 | 84 |
| Thumb extensors | 27.2 | 151 |
| Teres major | 26.4 | 129 |
| Abductor pollicis | 26.3 | 133 |
| Trapezius | 19.9 | 221 |
| Pectoralis major | 14.8 | 209 |
| Sternocleidomastoid | 7.2 | 181 |
| Paraspinal neck extensors | 1.5 | 198 |
Muscles affected
| Muscle . | Percentage affected . | Cases examined . |
|---|---|---|
| Infraspinatus | 71.8 | 216 |
| Serratus anterior | 70 | 230 |
| Supraspinatus | 65.7 | 204 |
| Biceps brachii | 61 | 223 |
| Rhomboids | 54.2 | 179 |
| Pronator teres | 52.3 | 155 |
| Brachioradialis | 48.1 | 162 |
| Wrist extensors | 47.4 | 209 |
| Deltoid | 46 | 226 |
| Triceps brachii | 43.4 | 221 |
| Wrist flexors | 36.3 | 212 |
| Finger extensors | 36.3 | 193 |
| Pronator quadratus | 33.3 | 45 |
| Deep flexors digits I and II | 30.6 | 180 |
| Dorsal interosseus | 30 | 217 |
| Adductor pollicis | 27.4 | 84 |
| Thumb extensors | 27.2 | 151 |
| Teres major | 26.4 | 129 |
| Abductor pollicis | 26.3 | 133 |
| Trapezius | 19.9 | 221 |
| Pectoralis major | 14.8 | 209 |
| Sternocleidomastoid | 7.2 | 181 |
| Paraspinal neck extensors | 1.5 | 198 |
| Muscle . | Percentage affected . | Cases examined . |
|---|---|---|
| Infraspinatus | 71.8 | 216 |
| Serratus anterior | 70 | 230 |
| Supraspinatus | 65.7 | 204 |
| Biceps brachii | 61 | 223 |
| Rhomboids | 54.2 | 179 |
| Pronator teres | 52.3 | 155 |
| Brachioradialis | 48.1 | 162 |
| Wrist extensors | 47.4 | 209 |
| Deltoid | 46 | 226 |
| Triceps brachii | 43.4 | 221 |
| Wrist flexors | 36.3 | 212 |
| Finger extensors | 36.3 | 193 |
| Pronator quadratus | 33.3 | 45 |
| Deep flexors digits I and II | 30.6 | 180 |
| Dorsal interosseus | 30 | 217 |
| Adductor pollicis | 27.4 | 84 |
| Thumb extensors | 27.2 | 151 |
| Teres major | 26.4 | 129 |
| Abductor pollicis | 26.3 | 133 |
| Trapezius | 19.9 | 221 |
| Pectoralis major | 14.8 | 209 |
| Sternocleidomastoid | 7.2 | 181 |
| Paraspinal neck extensors | 1.5 | 198 |
In most patients (59.8%) recovery of motor function began between the first and sixth month. A small group (7.9%) already reported to have noticed some recovery within 1–4 weeks. In 18.9% recovery set in after 6–12 months, in 8.5% after 1–2 years, and in 4.9% not until 2–3 years after the onset.
In 88.5% of the males and in 75.4% of the females clinical assessment revealed muscle atrophy during an attack; this difference was significant (P = 0.02). The median time for atrophy to first appear was 5 weeks; in 20% of the patients it was perceived as early as 2 weeks after the onset. Isolated long thoracic nerve palsy (in which serratus anterior atrophy is difficult to detect clinically) was ∼20 times as common in the 15.5% of patients without visible atrophy as it was in the group with atrophy (11.4 versus 0.5%, respectively). Six patients spontaneously mentioned fasciculations at onset in the later paretic muscles.
Sensory symptoms
Sensory symptoms during an attack were reported by 69.2% of the patients, and after clinical examination sensory involvement was found in 78.4%. Similar to atrophy, we found that in patients without sensory involvement, motor involvement of the upper plexus including the long thoracic nerve was more common (52%) than in those who did exhibit sensory symptoms (21.8%). Hypaesthesia was the most common complaint (45.9%), followed by a combination of paraesthesia and hypaesthesia in 39.2%, and paraesthesia only in 14.2%; only one patient (0.5%) reported allodynia. Paraesthesia usually occurred at the onset of an attack or afterwards with traction on the affected parts of the plexus (data not quantified).
The total of 39 different distribution patterns reported for sensory symptoms were regrouped into five anatomical regions as listed in Table 5. Ranging from dermatomes C3 and C4, or the lumbar region, to a combination of the axilla and fingertips, it is safe to say that ‘almost anything goes’ when it comes to the possible distribution patterns of sensory symptoms in NA. However, hypaesthesia and/or paraesthesia over the deltoid and lateral upper arm region were most common (48.9%). When sensory and motor deficits were recoded according to their truncal distribution within the plexus and compared, no correlation was found in the localization of the two affected modalities.
Distribution of sensory symptoms regrouped into anatomical regions
| Characteristic . | Percentage . | Cases . |
|---|---|---|
| (1) Lateral shoulder and/or arm | 48.9 | 87 |
| (2) Fingers or hand only | 20.8 | 37 |
| (3) Medial (fore)arm | 18 | 32 |
| (4) Neck, back and scapula | 5.6 | 10 |
| (5) Other | 6.7 | 12 |
| Characteristic . | Percentage . | Cases . |
|---|---|---|
| (1) Lateral shoulder and/or arm | 48.9 | 87 |
| (2) Fingers or hand only | 20.8 | 37 |
| (3) Medial (fore)arm | 18 | 32 |
| (4) Neck, back and scapula | 5.6 | 10 |
| (5) Other | 6.7 | 12 |
Distribution of sensory symptoms regrouped into anatomical regions
| Characteristic . | Percentage . | Cases . |
|---|---|---|
| (1) Lateral shoulder and/or arm | 48.9 | 87 |
| (2) Fingers or hand only | 20.8 | 37 |
| (3) Medial (fore)arm | 18 | 32 |
| (4) Neck, back and scapula | 5.6 | 10 |
| (5) Other | 6.7 | 12 |
| Characteristic . | Percentage . | Cases . |
|---|---|---|
| (1) Lateral shoulder and/or arm | 48.9 | 87 |
| (2) Fingers or hand only | 20.8 | 37 |
| (3) Medial (fore)arm | 18 | 32 |
| (4) Neck, back and scapula | 5.6 | 10 |
| (5) Other | 6.7 | 12 |
In more than half the patients (51.7%) there was no recovery of the sensory symptoms on follow-up. Moderate recovery was found in 19.6% and full recovery in 28.7%. In those who had fully recovered, recovery had set in between 0 and 3 months after onset in 19.5% (7.3% within the first week), after 3–6 months and 6–12 months in 29.3% each, and in 24% after 1–3 years.
Autonomical and other symptoms
In 32 patients (15.4%) signs of peripheral autonomic nervous system involvement, mainly the so-called distal vasomotor dysfunction (Low, 1997), were found in the areas with sensory or motor affection: vegetative and trophic skin changes, oedema at onset of the attack, temperature dysregulation, increased sweating and, rarely, changes in nail or hair growth (details not quantified). Autonomic dysfunction proved significantly more common in patients with motor involvement of the posterior cord, lower part or all parts of the brachial plexus, than in patients with upper and/or middle brachial plexus involvement (P = 0.006). Conversely, of all the patients who (also) had lower plexus involvement, 32.5% showed signs of vasomotor dysfunction. Almost all attacks with autonomic dysfunction were accompanied by sensory symptoms (93.8 versus 76.2% in attacks without autonomic dysfunction, P = 0.05). There was no correlation between the presence or absence of autonomic dysfunction and the distribution of pain or sensory symptoms. None of the patients exhibited a Horner's sign.
Of 99 patients 20 (20.2%) reported significant weight loss (>5 kg within a few weeks) during an attack. In this small group more people reported an antecedent event (68.4 versus 41.6%), which also more often was an infection (76.9%), than in people without weight loss (50%).
Predisposing factors and medical histories
In 53.2% of the attacks the patient reported an antecedent event. The nature of these events and their occurrence are presented in Table 6. Of note was that in females attacks during pregnancy or in the puerperium occurred in both INA and HNA patients. In 52.3% of the cases, the event occurred in the week preceding the attack; in 17.4% it occurred only hours before. The timing of the most common events versus the onset of the attacks is shown in Table 7. In the subgroup of 133 patients with only one untreated attack, the occurrence of an antecedent event yielded an average NRS pain score at onset that was 1.25 points higher than in patients without such an event (6.7 versus 7.9, P = 0.02).
Antecedent events
| Antecedent event . | Percentage . | Cases . |
|---|---|---|
| Infection | 43.5 | 50 |
| Exercise | 17.4 | 20 |
| Surgery | 13.9 | 16 |
| Peripartal* | 8.7 | 10 |
| Vaccination | 4.3 | 5 |
| Stress (psychological) | 4.3 | 5 |
| Trauma | 4.3 | 5 |
| Other† | 3.5 | 4 |
| Antecedent event . | Percentage . | Cases . |
|---|---|---|
| Infection | 43.5 | 50 |
| Exercise | 17.4 | 20 |
| Surgery | 13.9 | 16 |
| Peripartal* | 8.7 | 10 |
| Vaccination | 4.3 | 5 |
| Stress (psychological) | 4.3 | 5 |
| Trauma | 4.3 | 5 |
| Other† | 3.5 | 4 |
6 INA and 4 HNA patients; nine during puerperium, one in third trimester of pregnancy.
Two patients, after sleeping with their arm in an unusual position (PMP22 unknown, but otherwise typical attacks and no signs of pressure palsies on EMG examination), one after CVA, one during bed rest for lumbar HNP.
Antecedent events
| Antecedent event . | Percentage . | Cases . |
|---|---|---|
| Infection | 43.5 | 50 |
| Exercise | 17.4 | 20 |
| Surgery | 13.9 | 16 |
| Peripartal* | 8.7 | 10 |
| Vaccination | 4.3 | 5 |
| Stress (psychological) | 4.3 | 5 |
| Trauma | 4.3 | 5 |
| Other† | 3.5 | 4 |
| Antecedent event . | Percentage . | Cases . |
|---|---|---|
| Infection | 43.5 | 50 |
| Exercise | 17.4 | 20 |
| Surgery | 13.9 | 16 |
| Peripartal* | 8.7 | 10 |
| Vaccination | 4.3 | 5 |
| Stress (psychological) | 4.3 | 5 |
| Trauma | 4.3 | 5 |
| Other† | 3.5 | 4 |
6 INA and 4 HNA patients; nine during puerperium, one in third trimester of pregnancy.
Two patients, after sleeping with their arm in an unusual position (PMP22 unknown, but otherwise typical attacks and no signs of pressure palsies on EMG examination), one after CVA, one during bed rest for lumbar HNP.
Time to onset of attack per type of antecedent event
| Event . | Time to onset of attack and percentages . | . | . | . | Available cases . | |||
|---|---|---|---|---|---|---|---|---|
. | <24 h . | 1–7 days . | 1–2 weeks . | >2 weeks . | . | |||
| Infection | 8.2 | 65.3 | 16.3 | 10.2 | 49 | |||
| Exercise | 31.6 | 47.4 | 21.1 | 0 | 19 | |||
| Surgery | 37.5 | 50 | 6.3 | 6.3 | 16 | |||
| Peripartal | 11.1 | 55.6 | 22.2 | 11.1 | 9 | |||
| Event . | Time to onset of attack and percentages . | . | . | . | Available cases . | |||
|---|---|---|---|---|---|---|---|---|
. | <24 h . | 1–7 days . | 1–2 weeks . | >2 weeks . | . | |||
| Infection | 8.2 | 65.3 | 16.3 | 10.2 | 49 | |||
| Exercise | 31.6 | 47.4 | 21.1 | 0 | 19 | |||
| Surgery | 37.5 | 50 | 6.3 | 6.3 | 16 | |||
| Peripartal | 11.1 | 55.6 | 22.2 | 11.1 | 9 | |||
Time to onset of attack per type of antecedent event
| Event . | Time to onset of attack and percentages . | . | . | . | Available cases . | |||
|---|---|---|---|---|---|---|---|---|
. | <24 h . | 1–7 days . | 1–2 weeks . | >2 weeks . | . | |||
| Infection | 8.2 | 65.3 | 16.3 | 10.2 | 49 | |||
| Exercise | 31.6 | 47.4 | 21.1 | 0 | 19 | |||
| Surgery | 37.5 | 50 | 6.3 | 6.3 | 16 | |||
| Peripartal | 11.1 | 55.6 | 22.2 | 11.1 | 9 | |||
| Event . | Time to onset of attack and percentages . | . | . | . | Available cases . | |||
|---|---|---|---|---|---|---|---|---|
. | <24 h . | 1–7 days . | 1–2 weeks . | >2 weeks . | . | |||
| Infection | 8.2 | 65.3 | 16.3 | 10.2 | 49 | |||
| Exercise | 31.6 | 47.4 | 21.1 | 0 | 19 | |||
| Surgery | 37.5 | 50 | 6.3 | 6.3 | 16 | |||
| Peripartal | 11.1 | 55.6 | 22.2 | 11.1 | 9 | |||
The medical history of our patients appears to be a reasonable cross-section of the general population, judging from the prevalence of lumbar spine pathology (9.4%), malignancies (2.6%) and reported allergies (32.3%), which match that of the Dutch population (National Dutch compass for public health and health care, 2005). The prevalence of diabetes mellitus in our study population was 3% (6 INA, 1 HNA) versus 4% in the general population. In this small group, there were no patients with lumbosacral plexus pathology or weight loss during an attack. The mean age of onset in the 6 INA patients with diabetes mellitus was 57.4 years, and four of them had multiple attacks and two had had facial nerve involvement during an attack, which was not seen in other INA patients. We did not find other differences in course or outcome.
Ancillary investigations
Laboratory findings
Blood analyses were performed in 135 patients (54.9% of total) and in 27.8% of these cases one or more abnormalities were found. Most conspicuous were 10 patients with elevated liver enzymes in the early phase of their attacks, for which no explanation could be found (even after liver biopsy in two patients). Clinically, it concerned mostly middle-aged males (n = 8), with idiopathic NA (n = 9), suffering from bilateral (n = 8) and severe (n = 8) attacks which involved the phrenic nerve(s) and/or lumbosacral plexus in more than half of the cases (n = 5). DNA was tested for HNPP in 36 patients (14.6% of total); none were positive. We also found signs of a recent infection in 4.5%, a previous infection in 1.5%, mildly elevated creatine kinase in 1.5%, positive rheumafactors in 0.8% and other findings in 8%. In 9 out of 34 patients (26%; 5 males; 2 HNA patients) antiganglioside antibodies were found. Anti-Gm1 IgM was present in six, anti-Gm2 IgM in one; anti Gm1 and Gm2 IgG were (also) found in three patients each, and one patient had only anti-Gm3 antibodies (no type specification available). As our laboratory performs a qualitative analysis for antiganglioside antibodies (using immunostaining high performance thin-layer chromatography) titres were not available.
Lumbar puncture
CSF analysis was performed in 32 patients (13% of the total). One patient was tested because of a clinical diagnosis of viral meningitis, subsequently proven, occurring concurrently with the NA attack. In four other patients (12.5%) the CSF proved abnormal, with elevated protein in two, elevated protein with slight pleocytosis in one and oligoclonal bands (with an identical profile in serum) in one.
Electrodiagnostic tests
The most commonly used diagnostic test was an EMG examination, which was used in 214 (87%) of all patients. In 96.3% it was abnormal and compatible with NA (Suarez, 2005). Somatosensory evoked potential studies, performed in 11 patients, were abnormal in one: a male INA patient in whom the dermatomal SSEP of both arms showed a slowed and less well-defined response from the C5 root on the affected left side, while the C4, C6, C7 and C8 responses were within normal limits.
Imaging studies
In 85 patients (34.1% of total) a chest X-ray was performed, which was abnormal in 21 cases. We found no superior sulcus (Pancoast) lung tumour, but in 11 cases one (n = 8) or both (n = 3) diaphragm halves were elevated due to phrenic nerve involvement. In four patients we found signs of thus far undiagnosed obstructive pulmonary disease. Other findings included post-thoracotomy changes resulting from previous surgery (unrelated to the onset of symptoms), old sarcoidosis and old tuberculosis lesions.
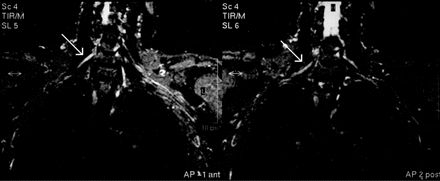
Many patients (n = 107, i.e. 43.5% of the total) also underwent an MRI scan of the cervical spine, which showed degenerative changes (including six cases with myelopathy) in more than half of the cases (52.8%). None of the abnormalities found was able to explain the clinical picture and course in these patients. Fifty patients (20.3% of total) also underwent an MRI scan of the brachial plexus, which was abnormal in three (6.3%), showing focal T2 hyperintensities in two and focal thickening of the plexus in one patient; the latter is shown in Fig. 1.
T2-weighted non-contrast MRI scan of the brachial plexus, showing a thickened and slightly hyperintense middle trunk on the right.
Functional recovery and employment
Untreated patients
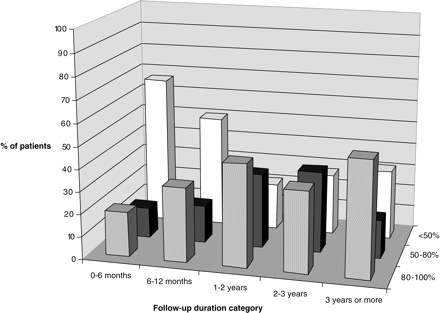
In 200 HNA and INA patients who had not received corticosteroid treatment, recovery parameters were grouped into five categories according to follow-up duration (0–6 months, 59 patients; 6–12 months, 56 patients, 1–2 years, 55 patients; 2–3 years, 19 patients; 3 years or more, 49 patients). Results are depicted in Table 8 and Figs 2, 3 and 4 (percentages in the category 2–3 years slightly skewed due to the smaller number of patients in this particular group). Overall, there were more females with a Rankin score of 3 (requiring some assistance in daily life) (16.9%) than males (3.4%) at the last follow-up visit. We found no correlation between age and outcome parameters such as muscle strength, sensory recovery, Rankin score or estimated recovery rate.
Subjective recovery rates and residual symptoms per duration of follow-up since last attack (HNA and INA, n = 200)
| Follow-up duration per category . | Full recovery according to patient (%) . | Unable to work (%) . | Rankin score (%) . | . | . | . | Cases . | |||
|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | 0 . | 1 . | 2 . | 3 . | . | |||
| 0 to <6 months | 0 | 56.2 | 0 | 18.7 | 75 | 6.3 | 42 | |||
| 6 to <12 months | 0 | 53.6 | 5.1 | 38.5 | 56.4 | 0 | 47 | |||
| 1 to <2 years | 3.8 | 30 | 2.4 | 35.7 | 54.8 | 7.1 | 45 | |||
| 2 to <3 years | 9.1 | 18.8 | 6.3 | 31.3 | 50 | 12.5 | 17 | |||
| 3 years or more | 7.7 | 26.7 | 7 | 34.9 | 51.2 | 7 | 49 | |||
| Follow-up duration per category . | Full recovery according to patient (%) . | Unable to work (%) . | Rankin score (%) . | . | . | . | Cases . | |||
|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | 0 . | 1 . | 2 . | 3 . | . | |||
| 0 to <6 months | 0 | 56.2 | 0 | 18.7 | 75 | 6.3 | 42 | |||
| 6 to <12 months | 0 | 53.6 | 5.1 | 38.5 | 56.4 | 0 | 47 | |||
| 1 to <2 years | 3.8 | 30 | 2.4 | 35.7 | 54.8 | 7.1 | 45 | |||
| 2 to <3 years | 9.1 | 18.8 | 6.3 | 31.3 | 50 | 12.5 | 17 | |||
| 3 years or more | 7.7 | 26.7 | 7 | 34.9 | 51.2 | 7 | 49 | |||
Subjective recovery rates and residual symptoms per duration of follow-up since last attack (HNA and INA, n = 200)
| Follow-up duration per category . | Full recovery according to patient (%) . | Unable to work (%) . | Rankin score (%) . | . | . | . | Cases . | |||
|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | 0 . | 1 . | 2 . | 3 . | . | |||
| 0 to <6 months | 0 | 56.2 | 0 | 18.7 | 75 | 6.3 | 42 | |||
| 6 to <12 months | 0 | 53.6 | 5.1 | 38.5 | 56.4 | 0 | 47 | |||
| 1 to <2 years | 3.8 | 30 | 2.4 | 35.7 | 54.8 | 7.1 | 45 | |||
| 2 to <3 years | 9.1 | 18.8 | 6.3 | 31.3 | 50 | 12.5 | 17 | |||
| 3 years or more | 7.7 | 26.7 | 7 | 34.9 | 51.2 | 7 | 49 | |||
| Follow-up duration per category . | Full recovery according to patient (%) . | Unable to work (%) . | Rankin score (%) . | . | . | . | Cases . | |||
|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | 0 . | 1 . | 2 . | 3 . | . | |||
| 0 to <6 months | 0 | 56.2 | 0 | 18.7 | 75 | 6.3 | 42 | |||
| 6 to <12 months | 0 | 53.6 | 5.1 | 38.5 | 56.4 | 0 | 47 | |||
| 1 to <2 years | 3.8 | 30 | 2.4 | 35.7 | 54.8 | 7.1 | 45 | |||
| 2 to <3 years | 9.1 | 18.8 | 6.3 | 31.3 | 50 | 12.5 | 17 | |||
| 3 years or more | 7.7 | 26.7 | 7 | 34.9 | 51.2 | 7 | 49 | |||
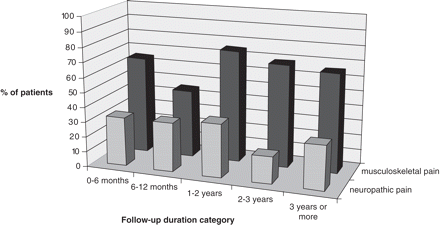
Percentage of patients with persisting pain for each of the five follow-up periods.
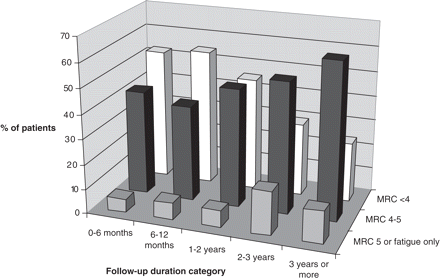
Percentage of patients with residual paresis for each of the five follow-up periods.
Estimated subjective overall recovery percentages according to the patients, for each of the five follow-up periods.
In the subgroup of 133 patients (125 INA, 8 HNA) who had suffered only one attack, pain at the most recent follow-up tended to be worse (average NRS score 2.4 points higher, 3.7 versus 6.1) in those who had distal vasomotor dysfunction (P = 0.06). The Rankin score was significantly higher in patients with distal vasomotor dysfunction (1.5 versus 2.0, P = 0.01) and in patients with joint contractures (1.6 versus 2.3; P = 0.01). It also tended to be slightly higher in females (1.5 versus 1.8; P = 0.07). Linear regression of recovery over time (defined in five time successive intervals) showed an increase of the subjective recovery percentages (also in categories) of 0.3 ‘category’ per time unit (P = 0.05), and also an increase in maximum MRC defined strength at follow-up of 0.2 ‘category’ per time unit (P = 0.001). Univariate and multivariate analyses yielded similar results.
The outcome results for INA (n = 39) and HNA (n = 10) patients with a long-term follow-up of three years or more were separated because, overall, both Rankin score and employment ability differed statistically between the two patient types. Many of these patients (INA 61.5%, HNA 80%) still had pain since their attack(s), with a tendency to a slightly higher mean NRS pain score in HNA patients (5.3 versus 3.7 in INA). In both groups a considerable proportion (INA 46.2%, HNA 62.5%) suffered from chronic pain. In the INA patients mild paresis was still present in 69.4% of cases, moderate paresis in 13.9% and severe paresis in 2.8%. The outcome for the HNA patients was worse: 40% had a mild paresis, 30% a moderate and 20% severe paresis. Most importantly, only two patients, i.e. 4.1% of the total and both with INA, had made a full recovery. As assessed by the patients themselves, 34.8% of those suffering from INA and 33.3% afflicted by HNA had made a 90–99% recovery. Conversely, 21.7% of INA patients reported <50% recovery, which was 66.7% for the HNA patients. The median Rankin score for patients with a follow-up of 3 years or more did not differ between HNA and INA; both groups had a score of 2, meaning that patients experienced some impairments in daily life but did not need help from others (van Swieten et al., 1988) In the INA group, 22.3% were unable to work and 36.8% had had to find a different type of job because of NA. Four HNA patients (40%) were unable to work, and two (20%) had changed jobs.
Corticosteroid-treated patients
There were 41 patients who had received open-label corticosteroid treatment for one of their attacks; in 29 it had been the attack that we further evaluated. The median time to the start of the treatment was 10 days, but the mean was 29.8 days (range 0–390 days). Dose regimens varied between patients; in only 18 the same schedule was followed, using a 2 week course of oral prednisone, 60 mg daily in the first week, and tapering the dosage by 10 mg per day (and 5 mg as a last step) in the second week. The median duration of treatment was 13 days (median 12.4, range 2–56 days). Side-effects were present in 8 of 26 patients (30.8%), and in 4 they were severe enough to stop using the corticosteroids. When asked whether they had noticed a favourable effect on their symptoms, 4 out of 20 patients responded ‘yes, definitely’, 9 said they'd experienced ‘some’ effect, 4 had only noticed a temporary relief, in 1 it had been ‘unclear’ and two reported no favourable effect whatsoever. The median time to a noticeable decrease in their initial pain in 19 patients was 5 days (mean 6.7, range 0–20 days), and the median time till any noticeable recovery of strength was 15 days (mean 29.8, range 0–130 days). In 3 out of 37, patients, a full documented recovery of their symptoms had taken place within 1 month after the onset; they had all started their treatment within 2 weeks after the onset (5, 6 and 12 days, respectively).
When statistically comparing the corticosteroid-treated patients with the untreated group, we found that subsequently treated patients had on average suffered more attacks (median 2 versus 1 in the untreated group; P < 0.001) and had a higher maximum NRS score during their treated attack (median 10 versus 9 in the untreated group; P = 0.02). No differences were found in other baseline variables such as age; sex; type of NA; handedness; onset symptom; duration of the initial pain; time till and severity of the initial paresis; and the occurrence of atrophy, sensory or vasomotor symptoms during the attack. When comparing outcome variables, the only statistically significant finding was the time until the start of paresis recovery, which was shorter in treated patients (28.2% within the first month versus 6.3% in the untreated group; P = 0.001). We could not detect a difference in other variables such as the duration of the initial pain, maximum present NRS score and use of analgesics, the occurrence of a chronic pain syndrome, maximum MRC level of strength recovery, complications such as frozen shoulder or shoulder dislocation, or Rankin score.
Discussion
Although the most common, typical phenotype of an attack in both HNA and INA is relatively well known (i.e. an acute very painful upper brachial plexopathy with a winged scapula), our data show that neuralgic amyotrophy is a syndrome with a broad range of clinical manifestations. Patients can present with isolated unilateral interosseus anterior nerve palsy and an inability to flex their index finger and thumb or a bilateral severe paresis of both upper extremities with severe orthopnoea due to phrenic nerve palsy—and anything in between. Painless attacks occur in a small minority (3.7%) and do not automatically mean that the patient is suffering from HNPP. The variability in the location and severity of symptoms most likely depends on exactly which parts of and the extent to which the plexus is affected by the presumed autoimmune process. From a clinical perspective, a ‘radicular’-type pain in the neck region or a pain provoked by passive movement or involvement of the paraspinal muscles on EMG examination does not exclude the disorder either. In addition, the disorder proved not to be limited to the brachial plexus; the lumbosacral plexus, phrenic nerve, intercostal nerves or recurrent laryngeal nerve could also be involved. This again (England, 1999) suggests that it is probably not accurate to describe NA as a pure brachial plexopathy and that from an anatomical perspective it would be more apt to call it a form of mononeuropathia multiplex—albeit with preferential involvement of the cervical roots, plexuses or nerves.
Besides the typical severe neuropathic pain at onset (with a NRS score ≥7 in 90%), the ‘patchiness’ of NA attacks remains a distinctive feature that will prompt the diagnosis in almost every case. There are also several limitations to the clinical phenotype: a perfectly symmetric involvement of both brachial (and/or lumbosacral) plexuses is very rare, as noted earlier (Tsairis et al., 1972), and even then it will not involve all peripheral nerves. A Horner's sign does not seem to belong to the NA phenotype either, although 15% of patients do exhibit symptoms of autonomic nervous system dysfunction and the actual symptom has been reported in one HNA patient (van Alfen et al., 2000). In practice its finding should warrant a prompt search for underlying structural plexus pathology.
Although motor symptoms are said to predominate in NA, almost 70% of the patients reported sensory symptoms in addition to the pain, and on examination hypaesthesic skin areas were found in almost 80%. This at least shows that the disorder itself does not seem to have a preference for affecting motor nerves. We came to a similar conclusion in an earlier histological study of HNA (van Alfen et al., 2005). The combination of our data and a previous report in the literature (Seror, 2004) suggest that attacks can also be either pure motor or pure sensory. The fact that paresis still dominates the clinical picture (over sensory symptoms) after the initial pain has disappeared could be explained by the predominance of proximal symptoms in most cases: the tactile spatial resolution is much lower on the back and shoulder than more distally in forearm or hands, and as the shoulder is not involved in any tactile process relatively large proximal hypaesthetic areas may go unnoticed in daily life. Our finding that the localization of motor symptoms does not correlate with that of the sensory loss seems to stress the multifocal character of NA lesions.
Of course the broad range of symptoms encountered in this study could be influenced by the fact that about a third of the patients were especially referred to our centre because of a somehow atypical clinical picture according to their referring physician. This may also have influenced the severity of symptoms found, as it seems likely that uncomplicated cases with good recovery within a few months would have a smaller chance of being referred for a second opinion. Considering the large number of patients that had first been diagnosed with another disorder, it may be even that very mild cases never get referred to a neurologist, but are simply treated as ‘aspecific shoulder pain’ in a primary care setting.
This study also confirms the predominance of male patients in both INA and HNA, which is perhaps remarkable considering that the attacks are presumed to be autoimmune in origin (in which case one would usually—but not always, as in Goodpasture's syndrome—expect a female predominance). The reason for this difference has not yet become evident. It might be that an unknown sex-specific factor in this disorder makes male patients more prone to the attacks. But alternatively it could also be that a persistent bias exists that makes male patients get referred more frequently for the analysis of their symptoms, for example because they are more dependent on physical strength to generate an income for the household.
A novel finding was that we found that the NA attacks quite often follow a course comprising three different consecutive types of pain. The acute and continuous neuropathic pain that heralds the onset of an attack is followed by a period of increased mechanical sensitivity of the damaged nerves, and subsequently a substantial number of patients suffer from musculoskeletal-type pains in both the affected and compensating muscles, especially at the site of their origin and insertion. The identification of these subsequent pain types and the finding that the continuous neuropathic pain at onset on average lasts for almost 4 weeks rather than 2 weeks as has often been assumed (Suarez, 2005) are important because in our experience the diagnosis is often questioned or discarded when the pain persists longer than expected. It is also important because many patients will seek and need medical advice for this complication. The acute pain responds best to a combination of a long-acting NSAID with a slow-release opiate, and the increased mechanical sensitivity sometimes needs treatment with co-analgesics. The musculoskeletal pain is usually hardly responsive to medication, and here physiotherapists tend to achieve the best results by having the patient maintain a fluent motion and normal daily use of the affected shoulder or limb, paying special attention to the so-called scapulothoracic and glenohumeral rhythms.
In contrast to previous reports (Suarez, 2005), we found abnormalities in over a quarter of the patients in whom additional laboratory tests had been performed. Although the results did not directly contribute to the diagnosis of NA, they generated two interesting findings. First, there were the grossly elevated liver enzymes for which no cause could be identified in 10, usually male, patients, most of whom seemed to have a clinical subtype of NA with extensive bilateral brachial plexus and phrenic nerve involvement. This subtype may be triggered by a specific but unknown antecedent infection that also causes a transient hepatitis. The future isolation of this potential infectious agent could shed more light on the autoimmune mechanism involved in NA. The second abnormality was the finding of antiganglioside antibodies in 26% of the patients tested. As previously reported by others (Suarez, 2005), we also found CSF abnormalities such as elevated protein, a slight pleiocytosis and oligoclonal bands in a few patients. The main implication of this finding is that NA can affect the peripheral nerves as proximally as the nerve root segments. This was also indicated by involvement of the paraspinal muscles in some patients.
The role of EMG in NA has been commented on by many researchers (for a summary see Suarez, 2005). Apart from facilitating the differentiation of NA and HNPP, we found the EMG served two additional purposes. First, the technique supplemented the clinical examination by confirming or excluding the involvement of certain peripheral nerves or parts of the plexus and secondly it contributed to an estimation of the degree of recovery, even when this was not yet clinically apparent to the patient or physician.
Imaging studies serve several purposes in NA. Chest X-rays in one-third of the patients, mainly requested to exclude a Pancoast tumour, did not find space-occupying lesions but revealed a diaphragmatic palsy in several patients. Plexus MRI scanning may be warranted in case of an initially progressive clinical picture or when recovery fails to occur. Results were occasionally abnormal in our series (3 of 50 patients), and the abnormalities found were possibly related to the focal inflammatory process, but otherwise non-specific. MRI scanning of the cervical spine in NA is controversial, because, although they were abnormal in more than half of the cases, the scans were always unable to explain the patient's clinical picture and course. Of course we did not evaluate all the patients who underwent an MRI scan for cervicobrachial pain during the period covered by our review, so we cannot say how many patients were finally diagnosed with a cervical radiculopathy. But our results show that any MRI finding should be interpreted with care and only in the light of a thorough history and physical examination to prevent patients from being misclassified as suffering from a cervical radiculopathy, which is liable if too much emphasis is placed on the radiological rather than the clinical picture.
We have shown that the course of an NA attack can be quite variable, with an acute, subacute or more insidious onset of symptoms, and with recovery setting in as early as within a month to no observable recovery after several years. We found no relationship between recovery and age. Group comparisons revealed that compared with INA patients HNA patients were prone to a more severe motor deficit. They also exhibited more functional disabilities on follow-up as reflected by their higher Rankin scores. In females, the initial neuropathic pain lasted shorter than in males. They more often had involvement of the lower and total brachial plexus, and had more functional impairments on follow-up.
Unexpectedly, in patients with idiopathic NA the recurrence rate after a first attack was much higher than previously reported: 26.1% versus an estimated 5% in the literature (Tsairis et al., 1972), even after a follow-up of only 6 years. This implies that the lifetime risk of recurrence could be even higher. Ofcourse a referral bias could also have influenced the number of recurrences found in this INA population, as we evaluated two-thirds of these patients only after their recurrence. It might also be that some of these patients actually suffered from HNA, but were unaware of affected family members. Nevertheless, 12% of the patients that we prospectively followed developed a recurrence within the first year, suggesting that once the diagnosis has been made a higher index of suspicion probably reveals a higher recurrence tendency in INA than hitherto assumed. Because the recurrence rate is much higher than the reported incidence of INA in the general population, the peripheral nervous system of INA patients also seems to have an underlying vulnerability for the immune-mediated attacks, analogous to what was hypothesized earlier for HNA (Klein and Windebank, 2005). HNA patients, however, were still found to suffer the most recurrences (74.5% of patients in this series), indicating that the genetic defect makes them even more prone to the attacks.
Fortunately, most of the patients that we reviewed tended to recover from a severe to a mild residual paresis in the course of months to a few years. This is still in contrast with many descriptions in the literature that usually report that 80–90% of the patients will have recovered after 2–3 years (Tsairis et al., 1972; Tonali et al., 1983; Cruz Martinez et al., 2002). This discrepancy can probably at least partly be explained by the differences in the definition of recovery used in the various reports. Recent studies have already reported less optimistic prognoses (England, 1999; van Alfen et al., 2000; Geertzen et al., 2000). Still, our case series shows that almost a third of patients suffer from chronic pain, and the majority of both INA and HNA patients exhibit persisting functional deficits after an average follow-up of >6 years. The recovery rate was slow in most patients, especially when bearing in mind that in the Netherlands sick employees are evaluated 6 and 12 months after the onset of their illness when it is officially determined whether they are legally fit to return to work. Such a definitive decision at that point in time is unrealistic for NA patients who may show further recovery for several more years. It is therefore recommended to the attending health professionals to communicate this and the patient's status to the evaluating authorities.
As to incidence, although with this study we did not set out to obtain data on a predefined population, we did see one fellow physician in our hospital (from a medical staff of 909) suffering from a new-onset NA each year during the course of the study. And, even if this is by no means a solid way of calculating the NA incidence rate, it does suggest that NA is (much) more common than what is generally assumed. Finally, in many patients it took quite some time to arrive at the proper diagnosis, with a median period to diagnosis of 10.5 weeks and with the mean time at a lengthy 43.8 weeks. The difficulty is probably due to an inability to recognize the clinical picture as that of NA, especially when patients present without the indicative winged scapula. In most patients, however, the typical pain at onset combined with a patchy paresis is pathognomonical for the diagnosis. An early identification may be essential, since it has been suggested that the attacks could be alleviated by immunomodulating therapy in the earliest stage (Suarez et al., 1996). Our very preliminary data on patients treated with open-label corticosteroids suggest that in some patients it is possible to favourably influence the course of the symptoms. We did not find a favourable effect on long-term outcome, but this may well be due to the large interindividual variability in symptoms and courses inherent to the NA phenotype, making it difficult to find effects on a group level, and being further complicated by the variability in treatment and follow-up assessment in this patient group. A randomized placebo-controlled trial evaluating the effect of oral prednisone in the acute phase is now under way in the Netherlands.
The authors are grateful for the kind cooperation of all the patients and referring physicians, especially our colleagues Prick, Gilhuis, Ostergaard, Leyten, Eekhof and Hoitsma.
References
van Alfen N, van Engelen BG, Reinders JW, Kremer H, Gabreels FJ. The natural history of hereditary neuralgic amyotrophy in the Dutch population: two distinct types?
van Alfen N, Gabreels-Festen AA, Ter Laak HJ, Arts WF, Gabreels FJ, van Engelen BG. Histology of hereditary neuralgic amyotrophy.
Beghi E, Kurland LT, Mulder DW, Nicolosi A. Brachial plexus neuropathy in the population of Rochester, Minnesota, 1970-1981.
Cruz-Martinez A, Barrio M, Arpa J. Neuralgic amyotrophy: variable expression in 40 patients.
England JD, Sumner AJ. Neuralgic amyotrophy: an increasingly diverse entity.
Geertzen JH, Groothoff JW, Nicolai JP, Rietman JS. Brachial plexus neuropathy. A long-term outcome study.
Klein CJ, Windebank AJ. Hereditary brachial plexus neuropathy. In: Dyck PJ, Thomas PK, editors. Peripheral neuropathy. Philadelphia: Elsevier Saunders;
Kuhlenbaumer G, Stogbauer F, Timmerman V, De Jonghe P. Diagnostic guidelines for hereditary neuralgic amyotrophy or heredofamilial neuritis with brachial plexus predilection. On behalf of the European CMT Consortium.
Kuhlenbaumer G, Hannibal MC, Nelis E, Schirmacher A, Verpoorten N, Meuleman J, et al. Mutations in SEPT9 cause hereditary neuralgic amyotrophy.
MacDonald BK, Cockerell OC, Sander JW, Shorvon SD. The incidence and lifetime prevalence of neurological disorders in a prospective community-based study in the UK.
National Dutch compass for public health and healthcare. Bilthoven: RIVM, Nationaal Kompas Volksgezondheid, versie 3.1,
Seror P. Isolated sensory manifestations in neuralgic amyotrophy: report of eight cases.
Suarez GA. Immune brachial plexus neuropathy. In: Dyck PJ, Thomas PK, editors. Peripheral neuropathy. Philadelphia: Elsevier Saunders;
Suarez GA, Giannini C, Bosch EP, Barohn RJ, Wodak J, Ebeling P, et al. Immune brachial plexus neuropathy: suggestive evidence for an inflammatory-immune pathogenesis.
van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients.
Tonali P, Uncini A, Di Pasqua PG. So-called neuralgic amyotrophy: clinical features and long term follow-up.
Tsairis P, Dyck PJ, Mulder DW. Natural history of brachial plexus neuropathy. Report on 99 patients.
Author notes
1Department of Neurology, Neuromuscular Centre Nijmegen and 2Clinical Neurophysiology, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands