Article Text
Abstract
Objective To compare non-invasive ventilation neurally adjusted ventilatory assist (NIV-NAVA) and non-invasive pressure support (NIV-PS) in preterm infants on patient–ventilator synchrony.
Design A randomised phase II crossover trial.
Setting Neonatal intensive care units of two tertiary university hospitals in Korea.
Patients Preterm infants born <32 weeks.
Intervention NIV-NAVA and NIV-PS were applied in random order after ventilator weaning. Data were recorded for sequential 5 min periods after 10 min applications of each mode.
Main outcome measures The electrical activity of the diaphragm (Edi), ventilator flow and pressure curves were compared to examine the trigger delay (primary outcome) and other parameters of patient–ventilator interaction (secondary outcomes) for each period.
Results Fifteen infants completed the protocol. Trigger delay (35.2±8.3 vs 294.6±101.9 ms, p<0.001), ventilator inspiratory time (423.3±87.1 vs 534.0±165.5 ms, p=0.009) and inspiratory time in excess (32.3±8.3% vs 294.6±101.9%, p=0.001) were lower during NIV-NAVA compared with NIV-PS. Maximum Edi (12.6±6.3 vs 16.6±8.7 μV, p=0.003), swing Edi (8.8±4.8 vs 12.2±8.7 μV, p=0.012) and peak inspiratory pressure (12.3±1.5 vs 14.7±2.7 cm H2O, p=0.003) were also lower during NIV-NAVA. The main asynchrony events during NIV-PS were ineffective efforts and autotriggering. All types of asynchronies except double triggering were reduced with NIV-NAVA. Asynchrony index was significantly lower during NIV-NAVA compared with NIV-PS (p<0.001). No significant differences in leakage, expiratory tidal volume or minute ventilation were observed, but the respiratory rate was lower during NIV-PS than during NIV-NAVA.
Conclusions NAVA improved patient–ventilator synchrony and diaphragmatic unloading in preterm infants during non-invasive nasal ventilation even in the presence of large air leaks.
Trial registration number Registered with http://www.clinicaltrials.gov (NCT01877720).
- Intensive Care
- Neonatology
- Respiratory
Statistics from Altmetric.com
What is already known on this topic?
Nasal intermittent positive pressure ventilation (NIPPV) supports breathing while avoiding invasive ventilation, which may cause lung damage in preterm infants.
Synchronised respiratory support delivery with the infant's inspiration is believed to increase the efficacy of NIPPV.
Neurally adjusted ventilatory assist (NAVA) has been shown to improve patient–ventilator synchrony during non-invasive ventilation (NIV).
What this study adds?
NAVA improved patient–ventilator synchrony and decreased diaphragm work of breathing during NIV in preterm infants even in the presence of large air leaks.
NAVA may be an optimal option for NIPPV in very preterm infants who are at highest risks of intubation and poor respiratory outcomes.
Introduction
Improvements in the survival of preterm infants have increased interest in non-invasive respiratory supports while avoiding invasive ventilation, which may cause lung damage. Although nasal intermittent positive pressure ventilation (NIPPV) appears to provide additional support for very preterm infants who cannot be managed using nasal continuous positive airway pressure alone, the benefits of NIPPV for prevention of bronchopulmonary dysplasia (BPD) remain unclear.1 ,2 In the meantime, because upper airway patency enables the transmission of pressure to the distal airways during inspiration, synchronised delivery of ventilatory cycles with infants’ inspiration is believed to increase the efficacy of NIPPV.3 It has been reported that patient–ventilator asynchrony is an important source of poor NIPPV tolerance, which in turn increases the NIPPV failure and further intubation.4 ,5 However, patient–ventilator synchrony is difficult to achieve in preterm infants receiving non-invasive ventilation (NIV). Whereas adults and children can use tight-fitting masks with minimal leakage, the nasal prongs or mask used in neonates often have large leaks. In addition, because preterm infants have high breathing rates and weak inspiratory efforts, patient–ventilator asynchrony during NIV is likely to be more frequent. However, few physiological studies have examined the complex interactions between patient inspiratory effort and ventilatory assist in preterm infants during NIPPV.
Neurally adjusted ventilatory assist (NAVA), which uses electrical activity of the diaphragm (Edi) to trigger, set the amount of pressure and cycle off the ventilator, has been shown to reduce asynchrony during NIV in a small number of low-birthweight infants6 and to maintain synchrony in rabbits despite high levels of leaks.7 However, data concerning NIV-NAVA in preterm infants are lacking, and physiological validation of patient–ventilator synchrony of NIV-NAVA is essential prior to clinical application in this population.
The aim of this crossover study was to compare NIV-NAVA and NIV-pressure support (PS) in terms of patient–ventilator synchrony in very preterm infants.
Methods
This randomised crossover trial was conducted from November 2013 to April 2014 in neonatal intensive care units of two tertiary university hospitals in Korea. The ethics committees of both institutions approved the protocol, and informed consent was obtained from parents of each patient.
Subjects
Intubated preterm neonates who were born at gestational age (GA) <32 weeks and mechanically ventilated for at least 48 h for respiratory distress were eligible if they were deemed to be ready for ventilator weaning with the following minimal ventilator settings for >12 h: (1) mean airway pressure ≤8 cm H2O, (2) peak inspiratory pressure ≤14 cm H2O, (3) fraction of inspiratory oxygen (FiO2) ≤0.4 and (4) mandatory respiratory frequency ≤35/min. The exclusion criteria were as follows: (1) major congenital anomalies, (2) use of sedatives or anaesthetics, (3) grade III or higher intraventricular haemorrhage, (4) phrenic nerve palsy or haemodynamic instability.
Study protocol
All patients were ventilated using a SERVO-i (Maquet Critical Care AB, Solna, Sweden). Before the study began, the standard feeding tube was replaced with a dedicated electrode-equipped catheter to detect Edi (Edi Catheter; Maquet Critical Care AB).
The SERVO-i was equipped with a specific NIV algorithm to compensate for leaks by automatically adjusting flow and triggers in NIV-PS. Studies of adults have demonstrated the benefit of NIV algorithms for patient–ventilator synchrony.8 ,9 The pneumatic inspiratory trigger in NIV-PS mode is based on a first-come-first-served system corresponding to either a pressure decrease of 1 cm H2O under the positive end-expiratory pressure (PEEP) or an expiratory flow decrease of 6 mL for 100 ms. The same PEEP was used during both NIV-PS and NIV-NAVA. The initial PEEP was set to 5–6 cm H2O, and the above PS level was set to 10–15 cm H2O.10 The NAVA level was adjusted to maintain similar levels of transcutaneous Pco2 (PtCO2), which was continuously measured using a SenTec Digital Monitor (SenTec AG, Therwil, Switzerland). Backup ventilation at a rate of 30/min was available if Edi was absent or apnoea occurred for more than 5–10 s. The upper pressure limit was set to 20–25 cm H2O.
Ventilation was applied via a Miniflow adaptor and neonatal binasal prongs or masks (Medin Medical Innovations, Olching, Germany) as appropriate to fit to infants’ nostrils or noses. A heated humidifier (Fisher & Paykel Healthcare, Auckland, New Zealand) was always used.
Each infant underwent two sessions of 15 min trials after a 5 min stabilisation following extubation. NIV-PS and NIV-NAVA were consecutively applied in a random order, determined by a block randomisation method on a specified website. The 10 min period after changing the mode was considered to be a washout period. Data from the last 5 min of each session were recorded and stored on a dedicated personal computer. One of the investigators observed the patient bedside throughout the entire study period. Heart rate (HR), respiratory rate (RR), oxygen saturation (SpO2) and PtCO2 were monitored continuously. Blood pressure was measured non-invasively every 5 min, and infantograms were obtained before and after the study to monitor pulmonary or gastrointestinal adverse events.
The protocol was discontinued if any of the following problems developed: a required increase in FiO2 >0.2 to maintain SpO2 >88%, RR >80/min or HR >200/min.
Data acquisition and measurements
The delivered pressure, flow, ventilator rate of cycling, tidal volume, minute ventilation, leakage and Edi were acquired from the ventilator through an RS232 interface at a sampling rate of 100 Hz. These data were recorded using the dedicated software Servo Tracker V.4.2 (Maquet Critical Care AB). To determine the neural inspiratory time (Tineural), ventilator inspiratory time (Tiventilator), inspiratory time in excess (Tiexcess), trigger delay (Td) and asynchrony events, a breath-by-breath analysis of simultaneous pressure, flow and Edi curves was performed using customised software based on Microsoft Excel (Microsoft, Redmond, Washington).
The primary outcome, Td was defined as the time difference between the initial increase in Edi signal and the beginning of inspiratory flow delivered by the ventilator.11 Tineural was defined as the time interval between the initial increase and the maximum value of Edi. Tiventilator was defined as the time interval between the beginning and the end of inspiratory flow, and Tiexcess (%) was calculated as ((Tiventilator−Tineural)/Tineural)×100.12 ,13 The amplitude of the swing in Edi (swing Edi) was calculated by subtracting the baseline Edi from the peak Edi, which represented the patient's neural respiratory drive and/or effort.14 ,15
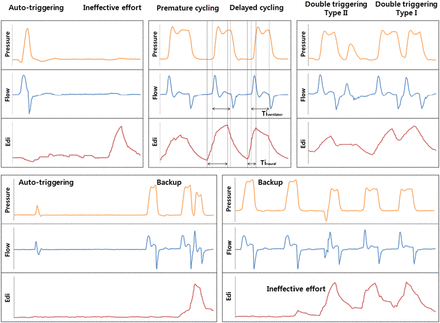
As secondary outcomes, five main types of asynchrony events were quantified according to previously published definitions (figure 1).12 ,13 Backup ventilator cycles were excluded from the analysis of asynchrony events. To estimate the extent of asynchrony, we calculated the asynchrony index (AI), which is the total number of asynchrony events divided by the sum of the ventilator cycles and ineffective efforts, expressed as a percentage.13 ,16
Representative tracings of asynchrony events and backup ventilation. Autotriggering, a cycle delivered by the ventilator in the absence of an Edi signal; ineffective effort, an inspiratory Edi signal not followed by an assisted ventilator cycle; premature cycling, a cycle with a Tiventilator < Tineural; delayed cycling, a cycle with a Tiventilator >2×Tineural; double triggering type I, two ventilator cycles associated with a biphasic Edi signal; double triggering type II, two ventilator cycles during the same inspiratory Edi signal. Edi, tracing of the electrical activity of the diaphragm; Tiventilator, ventilator inspiratory time; Tineural, neural inspiratory time.
Statistical analysis
On the basis of results obtained in a previous study,6 ,17 we assumed that in preterm infants, the mean Td values were 76 ms in NIV-NAVA and 112 ms in NIV-PS, with a mean SD of 33 ms. The sample size required for achieving significance in a paired t test with 80% power and 5% significance was calculated to be 14 infants. Therefore, assuming a 10% dropout rate, we estimated that 16 subjects would be needed.
We defined the validation data as per-protocol analyses. Statistical analysis was performed using SPSS V.21.0 (SPSS, Chicago, Illinois, USA). After testing for the normal distribution of each ventilator variable, paired t tests or Wilcoxon signed-rank tests were used.
Results
Of 16 preterm infants enrolled, one patient was discontinued because of sustained bradycardia in NIV-PS mode (figure 2). The patient was reintubated shortly after termination of the protocol. Remaining 15 infants completed the 30 min crossover comparison. The interfaces used were binasal prongs in nine infants and a nasal mask in six infants. The patients’ baseline characteristics and ventilator settings are summarised in table 1.
Clinical characteristics of the patients and ventilator settings
Study profile. aThis infant was excluded from the study because of an episode of sustained bradycardia during NIV-PS mode. bNo reliable data were available because this baby was reintubated after discontinuation of the study. NIV-NAVA, non-invasive neurally adjusted ventilatory assist; NIV-PS, non-invasive ventilation pressure support.
Main respiratory outcomes
As shown in table 2, mean values of Td, Tiventilator and Tiexcess were significantly shorter during NIV-NAVA than NIV-PS. There were greater variations in Td (range 116.6–425.5 vs 20.9–48.8 ms, p<0.001) and Tiventilator (range 245.9–845.0 vs 300.7–650.6 ms, p=0.031) during NIV-PS than NIV-NAVA. Tineural did not differ between the two modes. Maximum Edi, swing Edi and peak inspiratory pressure were lower during NIV-NAVA compared with NIV-PS. Pneumatic RR was higher during NIV-NAVA compared with NIV-PS. Mean airway pressure, minute ventilation, expiratory tidal volume and level of leakage did not differ between the two modes.
Comparison of two methods of ventilation
Asynchronies
The total number of asynchrony events was significantly lower during NIV-NAVA compared with NIV-PS (table 2). The main asynchrony events during NIV-PS were ineffective efforts and autotriggering, and these two types of asynchrony were reduced during NIV-NAVA. Accordingly, AI was significantly lower during NIV-NAVA. AI specific to leakage, which only integrated leak-related asynchronies, namely ineffective efforts, autotriggering and delayed cycling,9 was calculated to have a median value of 70.4% (IQR 67.8%–74.6%) during NIV-PS.
No problems related to the insertion of the Edi catheters occurred. Blood pressure, HR, RR, SpO2 and PtCO2 did not vary substantially within patients throughout the study. Eight adverse events occurred: six hypoxaemia (SpO2 <80%) and one bradycardia (HR <100/min) during NIV-PS, and one hypoxaemia during NIV-NAVA. None of the above events lasted longer than 2 min, except the episode of bradycardia during NIV-PS, which occurred in the patient who was withdrawn and reintubated. No events were associated with pulmonary air leak, ileus or gastrointestinal perforation.
Discussion
This is the first randomised crossover study to implement NIV-NAVA in very preterm infants (GA range of 23+6–29+4 weeks and birth weight range of 500–1480 g) who face the greatest risks of BPD and poor respiratory outcomes. Despite our inclusion criteria was GA <32 weeks, there was no subject who had been mechanically ventilated for >48 h among preterm infants of GA 30 to <32 weeks. Based on the finding that the median duration of prior ventilation was 21 days, most of the included patients may well have been on the way to developing BPD.
Consistent with previous work in adults and children, we found that neurally triggered and cycled non-invasive support shortened Td and improved patient–ventilator synchrony in preterm infants. However, the level of leakage that we observed in this study (87.6% in NIV-NAVA and 86.7% in NIV-PS) was higher than levels previously reported in adults and children (10%–43%).16 ,18–20 This discrepancy may be due to the difficulty of tightly fitting the nasal prongs or masks and leakage from the mouth during NIV. Because NIV-NAVA is theoretically unaffected by this high level of leakage, the benefits of NIV-NAVA for patient–ventilator synchrony might be well marked in this population.
In present study, Td, Tiventilator and Tiexcess were significantly lower during NIV-NAVA compared with NIV-PS. These results are most likely due to the synchronised simultaneous increase in airway pressure in NIV-NAVA. Poorly synchronised NIPPV increases in inspiratory time during spontaneous breathing in preterm infants have previously been reported.1 There is also evidence that distortion of the preterm infant's soft chest wall during inspiration may be attenuated by well-synchronised NIPPV.21 ,22 Although work of breathing was not measured in our study, because both the maximum Edi and swing Edi levels decreased during NIV-NAVA, we can infer that improved synchronisation during NIV-NAVA promotes diaphragm unloading.15 ,23
A decrease in RR during NIV-PS compared with NIV-NAVA (33.3±13.1 vs 46.3±12.6/min, p=0.002) was noted; the former is similar to the rate of backup ventilation, and the latter is similar to the normal breathing rate of premature infants. This finding may suggest that infants’ own respiratory efforts may be suppressed by poorly synchronised respiratory support. Although it is unclear how spontaneous breathing and NIPPV pressure interact in neonates, one observational study of non-synchronised NIPPV suggested that infants became entrained to non-synchronised NIPPV cycles.24 As shown in figure 3, respiratory supports during NIV-PS were delivered after the peak of Edi; assistance may have continued while a new neural respiratory cycle was starting, which in turn, was not triggered. As another explanation of the lower RR during NIV-PS, the higher PS during NIV-PS (14.7±2.7 vs 12.3±1.5 cm H2O, p=0.003) might have suppressed the respiratory centre via feedback control of the Hering–Breuer reflex.
Time tracing of the sample pressure, flow and electrical activity of the diaphragm (Edi) over a period of 3 s, illustrating the differences between non-invasive neurally adjusted ventilatory assist (NIV-NAVA) and non-invasive ventilation pressure support (NIV-PS). The subject of this sample was a girl at a postnatal age of 23 days (postmenstrual age of 32+6 weeks) weighing 1600 g who was born at gestational age 29+4 weeks with a birth weight of 1305 g (A, during NIV-NAVA; B, during NIV-PS). The dark area represents trigger delay, and the downward-pointing arrow indicates an ineffective effort.
There have been concerns that NIPPV may result in increased intrathoracic pressure, resulting in a risk of pneumothorax or bowel perforation.25 ,26 Although no adverse events of air leakage or gastrointestinal perforation occurred in this study, when an infant's own breathing conflicts with mechanical breaths during non-synchronised pressure delivery, the resulting increase in transpulmonary pressure could lead to a higher risk of barotrauma and ventilator-induced lung injury.
During NIV-NAVA, a significant decrease in asynchrony events occurred, except double triggering, compared with NIV-PS. Type I double triggering was only observed during NIV-NAVA, and was responsible for the difference in double triggering between the two modes. Although type II double triggering corresponds to genuine asynchrony, in which one neural inspiration triggers two pneumatic cycles, type I double triggering is not strictly considered a patient–ventilator asynchrony because it is the consequence of a biphasic Edi signal.27
However, regardless of the use of NIV-NAVA or NIV-PS, the overall AI of this study was higher than that previously reported in children or adults (2%–11% during NIV-NAVA and 10%–66% during NIV-PS).5 ,11 ,16 Although AI >10% is usually considered severe in adults, most of the infants included in this study exhibited AI >10%, even during NAVA. This reflects how difficult it is to achieve patient–ventilator synchrony in preterm infants during NIV.
Several limitations of this study should be considered. First, this study was performed using a single ventilator, SERVO-I, equipped with an internal flow sensor, which may not be ideal for triggering and delivering NIV-PS in preterm infants.11 More decisive results could be obtained from additional comparative studies using another ventilator to deliver NIPPV, particularly a ventilator equipped with another sensor system, such as a proximal flow sensor or an external sensor capsule. Second, although we set the NAVA level to maintain similar levels of PtCO2 throughout the entire study period, we cannot ensure whether the level of assistance was optimal for each patient or that was actually similar between the two modes. Third, although we chose relatively high cycling-off criteria of 50%–70% in NIV-PS to increase the sensitivity of detection of patient respiration during leakage, in some cases, this might have led to short inspiratory PS times and aggravated premature cycling during NIV-PS.
Although this study was a physiological study, and cannot provide any conclusive statements about the clinical usefulness of NIV-NAVA, it demonstrates the clear potential of how Edi can help to overcome the current limitations in synchronising assistance to preterm infants during NIV. In addition, our findings suggest that pressure transmission during NIPPV is improved by synchronisation through NAVA. Additional large trials are required to determine whether NIV-NAVA may help to improve respiratory outcomes in target populations.
Conclusions
This study demonstrated that, compared with NIV-PS, NIV-NAVA significantly reduced Td, Tiventilator, Tiexcess and asynchrony events. Most importantly, in the presence of large air leaks during NIV, NAVA improved patient–ventilator synchrony and decreased maximum and swing Edi, which represent the patient's work of breathing. NAVA may, therefore, be an optimal option for NIPPV in preterm infants. However, large clinical trials in this population are needed to address clinical evidence that improved patient–ventilator synchrony via NIV-NAVA results in improved outcomes.
Acknowledgments
We thank the members of the Medical Research Collaborating Centre at the Seoul National University Hospital for assistance with the statistical analysis and advice. The contributions of the nurses and medical staff members of the Seoul National University Children's Hospital and Seoul National University Bundang Hospital neonatal intensive care units are gratefully acknowledged; we are particularly grateful to You Jung Lim who provided valuable professional assistance in conducting this clinical trial.
References
Footnotes
Contributors JL conceptualised and designed the study, carried out the initial analyses and drafted the manuscript. H-SK conceptualised and designed the study, and reviewed and revised the manuscript. YHJ collected data and participated in the data analysis. SHS participated in conducting the study and analysing the data. E-KK participated in the design of the study and revised the manuscript. CWC participated in the design of the study and revised the manuscript for important intellectual content. BIK conceived of the study, participated in coordinating the study and revised the manuscript for important intellectual content. J-HC conceived the study, participated in coordinating the study and revised the manuscript for important intellectual content. All authors read and approved the final version of the manuscript.
Funding This study was funded by The Rotary Korea (Seoul, Korea), and was partly supported by Maquet Critical Care AB (Solna, Sweden) with respect to technical assistance with data acquisition and the provision of Edi catheters for the subjects included in this study.
Competing interests None declared.
Patient consent Obtained.
Ethics approval Seoul National University Hospital Institutional Review Board, Korea.
Provenance and peer review Not commissioned; externally peer reviewed.