Article Text
Abstract
Pharyngeal pressures in 11 preterm infants, receiving binasal Hudson prong continuous positive airway pressure (CPAP) pressurised by bubbling bottles, were measured. The mean (95% confidence interval) pressure drop from the prongs to the pharynx was 3.2 (2.6 to 3.7) cm H2O with mouths open and 2.2 (1.6 to 2.8) cm H2O with mouths closed. Mouth closure augments CPAP transmission.
- 95% CI, 95% confidence interval
- CPAP, continuous positive airway pressure
- Fio2, fractional inspired oxygen
- FG, French gauge
- IQR, interquartile range
- NICU, neonatal intensive care unit
- RDS, respiratory distress syndrome
- continuous distending pressure
- pharyngeal manometry
- continuous positive airway pressure (CPAP)
- premature infant
Statistics from Altmetric.com
- 95% CI, 95% confidence interval
- CPAP, continuous positive airway pressure
- Fio2, fractional inspired oxygen
- FG, French gauge
- IQR, interquartile range
- NICU, neonatal intensive care unit
- RDS, respiratory distress syndrome
- continuous distending pressure
- pharyngeal manometry
- continuous positive airway pressure (CPAP)
- premature infant
Continuous positive airway pressure (CPAP) is an effective treatment for preterm infants with respiratory distress syndrome or apnoea or after extubation.1 Our previous research showed that short binasal prongs deliver the pressure most effectively because of low resistance.2 Whichever nasal device or pressure generating system is used, it is the pressure transmitted to the airway that is important.
The optimum CPAP for infants with various degrees of lung disease remains uncertain. It is also unknown if small differences in CPAP transmission to the airway are clinically important. The pressure in the upper airway is an important measure of how much pressure is transmitted from the CPAP system. Chilton and Brooks3 measured pharyngeal pressures in infants treated with nasal CPAP via Argyle prongs (Sherwood Medical, St Louis, Missouri, USA). The tips of non-perfused, air filled catheters were placed in the pharynx, and the end expiratory pressure measured over just five breaths. They found that, when the mouth was open, pharyngeal pressure was about 48±4% below that in the prong. However, this was not a consistent finding, as in some infants the pharyngeal pressure at end expiration was observed to fall with imposed mouth closure.
It has been suggested that ensuring that a baby’s mouth is kept closed during nasal CPAP increases its effectiveness.4 The aim of this project was to characterise the pharyngeal pressure in preterm infants treated with CPAP via Hudson prongs (Hudson-RCI, Temecula, California, USA) pressurised by an underwater bottle system, and to accurately assess the effect of mouth closure.
METHODS
Measurement of pharyngeal pressure
A 6 French gauge, multi-lumen, soft, silastic catheter (Dentsleeve Pty Ltd, Adelaide, Australia) was designed so that the openings of two air perfused lumens could be positioned in the pharynx.5 The lumens for measuring pressure were 0.35 mm in diameter and their openings 1 cm apart. They were air perfused at 2 ml/min to keep the holes clear. The catheter was inserted through the mouth (seven infants) or nose (four infants). Positioning of the openings in the pharynx was aided by observing a high pressure when the distal opening entered the upper oesophageal sphincter and then the catheter was withdrawn slightly. Although nasal catheter placement permitted more stable recordings, it may have influenced nasal resistance and air leak. The catheter length enabled gastric feeds through a larger central lumen (diameter 0.75 mm).
Pressure transducers (Sensym; Sensortechnics, Puchheim, Germany) measured pressure (range 0–13 cm H2O) at the external ends of the two channels. The output was amplified (Applied Measurement, Melbourne, Australia), digitised, and recorded using Spectra Physiological Software (Grove Medical Ltd, Hampton, UK, version 3.004) at 200 Hz and observed in real time. Before each study, the system was calibrated to a water manometer and the output offset to zero after air perfusion.
Pressure response characteristics
The system’s pressure response characteristics were assessed by comparing its output with that recorded by a transducer connected directly to a pressure test chamber. As expected, there was amplitude attenuation. Damping was 15% when a test pressure of 10 cm H2O was applied at 1 Hz and increased considerably with increasing frequency. Importantly, the measure of interest, the mean pressure, did not vary from that recorded by the reference transducer by more than ±0.1 cm H2O, irrespective of the frequency.
The CPAP system
Hudson prongs pressurised with the Fisher and Paykel (Auckland, New Zealand) underwater bubble CPAP device were used in this study, as this is the preferred system used at the Royal Women’s Hospital, Melbourne.
Mouth position
Pharyngeal pressures were measured with the infant’s mouth in both the passive and actively closed positions with pressures from the CPAP device of 3–8 cm H2O. Set pressures were altered by 1 cm H2O increments to ensure clinical stability. The position designated as passive involved no direct measures to close the mouth during the measurement. Active mouth closure was achieved with gentle pressure applied under the infant’s chin with a single finger.
Pressure in the Hudson prong was measured concurrently with a Sensym pressure transducer. Mean pharyngeal pressure measurements were taken from the longest segment of stable recording for a minimum of 20 seconds and when the system was bubbling.
A paired samples t test with 95% confidence limits was used to compare differences.
Study population
This was an observational study on a convenience sample of stable preterm infants receiving nasal CPAP in the neonatal intensive care unit of the Royal Women’s Hospital, Melbourne, Australia in 2001. The study was approved by the Royal Women’s Hospital research and ethics committee, and informed parental consent was obtained.
RESULTS
The parents of 47 infants were approached for consent; 28 declined. Results from the first eight infants studied were excluded because of unacceptable error in the original amplifier (Synectics Medical, Stockholm, Sweden). The replacement amplifier was highly accurate. Results are presented for the remaining 11 infants where the replacement amplifier was used. Their median age was 14 days (interquartile range 12 to 46) with a mean (SD) corrected gestational age of 30.6 (1.9) weeks and a mean (SD) weight of 1151 (269) g.
Figure 1 shows a recording from an infant exhibiting pressure change with mouth closure. It shows two channels of pharyngeal pressure and the pressure from the Hudson prong. The pressure in both channels recording pharyngeal pressure was virtually identical.
A 36 second recording of two pharyngeal pressures and the pressure in the Hudson prong nasal continuous positive airway pressure (CPAP) device from an infant with a birth weight of 1520 g receiving a nasal CPAP of 7 cm H2O at a flow of 8 litres/min. During the first 10 seconds, the infant’s mouth is open, then during the next 22 seconds it is closed, and in the last 4 seconds it is open. The pressure in the Hudson prong shows variation because it is pressurised by underwater bubbling. The amplitude increases with mouth closure, indicating less gas leak from the system and faster underwater bubbling. The two pharyngeal pressures are almost superimposed. The rhythmical pressure variation is due to infant breathing.
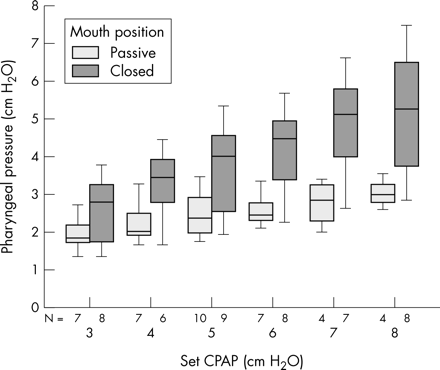
The pharyngeal pressures with the mouth in the actively closed and passive positions at different set pressures are presented as a box plot (fig 2). The increase in pharyngeal pressure with mouth closure, and a trend for the difference in pharyngeal pressure between closed and passive postures to increase with increasing set pressures from the CPAP device is apparent. The mean pharyngeal pressure always increased for each 1 cm H2O increment in CPAP when the mouth was closed or passive. Not all of these incremental differences were significant. However, the number of paired values available for incremental pressure comparisons was small.
Set pressure versus pharyngeal pressure in passive and closed mouth positions. This shows the effect on pharyngeal pressure at continuous positive airway pressures (CPAPs) of 3–8 cm H2O with the mouth open or closed. The boxes represent the interquartile range with the horizontal line at the median value. The whiskers show the 95% confidence intervals. N = number of observations.
The overall mean drop in pressure from the Hudson prong to the pharynx, with the mouth in either a passive or closed position, was 2.6 cm H2O (95% CI 2.2 to 2.9) (p<0.05). The mean drop in pressure from the Hudson prong to the pharynx with the mouth in the passive position was 3.2 cm H2O (95% CI 2.6 to 3.7) (p<0.05). When the mouth was actively closed, the mean drop in pressure from the Hudson prong to the pharynx was 2.2 cm H2O (95% CI 1.6 to 2.8, p<0.05). The mean difference between pharyngeal pressure in the passive and closed postures was 1.1 cm H2O (95% CI 0.7 to 1.4) (p<0.05). When the results for CPAPs of 3–4 cm H2O are excluded, because they are not often used in our unit, the mean difference between pharyngeal pressure in the passive and closed postures is only slightly higher at 1.2 cm H2O (95% CI 0.8 to 1.7) (p<0.05).
DISCUSSION
Obtaining highly accurate pharyngeal pressure measurements over prolonged periods in preterm infants receiving CPAP was difficult. Artefacts due to swallowing, movement, and secretions in the measuring catheters commonly rendered the recordings uninterpretable. However, satisfactory recordings were possible when the infants were quiet.
The results show that the prong pressure is not all transmitted to the pharynx, but is more effectively transmitted when the mouth is actively closed. There is considerable variation between infants in the pharyngeal pressure rise with mouth closure. This is predominantly due to variation in leak around the prongs at the nostrils. Air leak from between the lips also occurs despite jaw closure.
The mean pharyngeal pressure was never higher than the delivered pressure. This suggests that, with this CPAP system, there is always some pressure loss regardless of mouth position. We conclude that babies are unlikely to expire through the CPAP device. If this is true with other devices, then nasal CPAP is unlikely to increase expiratory resistance.6
Some neonatal intensive care units use devices such as chinstraps and pacifiers to reduce mouth leak. However, many babies receiving nasal CPAP benefit even though their mouths are open. Clinicians should be aware that pressures transmitted to the airway may be very low when the mouth is not actively closed, particularly at low set pressures.
We speculate that pharyngeal pressures observed in preterm infants in this study (median age 14 days) are likely to be similar in those with respiratory distress syndrome in the first days of life. However, our results do not permit conclusions on the safety and effectiveness of active mouth closure during nasal CPAP.
Acknowledgments
Mr Ed Hingeley, Biomedical Engineer, Royal Women’s Hospital, Melbourne, Australia. Professor John Dent, Professor of Gastroenterology, Royal Adelaide Hospital, Australia. Dr Taher Omari, Scientist, University of Adelaide, Australia. Nursing staff, babies, and parents of the neonatal intensive care unit of the Royal Women’s Hospital, Melbourne, Australia.
Footnotes
-
AGDeP was supported by the Royal Women’s Hospital Foundation and the Division of Research and Education, Royal Women’s Hospital, Melbourne. PGD was supported by an NHMRC Practitioner Fellowship, Murdoch Children’s Research Institute.
-
Competing interests: none declared