Article Text
Abstract
Background The effect on Paco2 of high concentration oxygen therapy when administered to patients with severe exacerbations of asthma is uncertain.
Methods 106 patients with severe exacerbations of asthma presenting to the Emergency Department were randomised to high concentration oxygen (8 l/min via medium concentration mask) or titrated oxygen (to achieve oxygen saturations between 93% and 95%) for 60 min. Patients with chronic obstructive pulmonary disease or disorders associated with hypercapnic respiratory failure were excluded. The transcutaneous partial pressure of carbon dioxide (Ptco2) was measured at 0, 20, 40 and 60 min. The primary outcome variable was the proportion of patients with a rise in Ptco2 ≥4 mm Hg at 60 min.
Results The proportion of patients with a rise in Ptco2 ≥4 mm Hg at 60 min was significantly higher in the high concentration oxygen group, 22/50 (44%) vs 10/53 (19%), RR 2.3 (95% CI 1.2 to 4.4, p<0.006). The high concentration group had a higher proportion of patients with a rise in Ptco2 ≥8 mm Hg, 11/50 (22%) vs 3/53 (6%), RR 3.9 (95% CI 1.2 to 13.1, p=0.016). All 10 patients with a final Ptco2 ≥45 mm Hg received high concentration oxygen therapy, and in five there was an increase in Ptco2 ≥10 mm Hg.
Conclusion High concentration oxygen therapy causes a clinically significant increase in Ptco2 in patients presenting with severe exacerbations of asthma. A titrated oxygen regime is recommended in the treatment of severe asthma, in which oxygen is administered only to patients with hypoxaemia, in a dose that relieves hypoxaemia without causing hyperoxaemia.
Clinical trial number ACTRN12607000131459.
- Carbon dioxide
- oxygen
- asthma
- randomised controlled trial
Statistics from Altmetric.com
Key messages
What is the key question?
Does high concentration oxygen therapy cause an increase in Paco2 in severe exacerbations of asthma?
What is the bottom line?
High concentration oxygen therapy causes a clinically significant increase in Ptco2 in patients presenting with severe exacerbations of asthma.
Why read on?
In the treatment of severe asthma it is recommended that oxygen is administered only in patients with hypoxaemia, in a dose that relieves hypoxaemia without causing hyperoxaemia.
Introduction
It is well recognised that high concentration oxygen therapy may lead to carbon dioxide (CO2) retention when administered to patients with acute exacerbations of chronic obstructive pulmonary disease (AECOPD)1 2 and that worsening ventilation–perfusion mismatch due to release of hypoxic pulmonary vasoconstriction with a resulting increase in physiological dead space is one of the major mechanisms causing this effect.3–7 In contrast, the risks and benefits of oxygen therapy in severe exacerbations of asthma are less well understood. As with AECOPD, the main gas exchange abnormality in severe exacerbations of asthma is ventilation–perfusion mismatch,8–10 and oxygen administration has been shown to worsen the degree of mismatch.10–15 There are preliminary data from case reports, case series and a single randomised controlled trial to suggest that high concentration oxygen therapy may potentially lead to CO2 retention in severe exacerbations of asthma.10–12 16–21 However, there are no randomised controlled trials comparing high concentration oxygen therapy with a titrated oxygen regime, in which oxygen is administered only to patients with hypoxaemia, to relieve hypoxaemia but avoiding hyperoxaemia, as recommended in recent guidelines.22–24
In this randomised controlled trial we investigated the effects of high concentration oxygen therapy on Paco2 in patients presenting to the Emergency Department (ED) with severe exacerbations of asthma. A comparison was made with oxygen therapy titrated as required to relieve hypoxaemia, with a target oxygen saturation of between 93% and 95%. The current study was designed to test the hypothesis that uncontrolled high concentration oxygen would result in an increase in the Paco2 compared with the titrated oxygen regime.
Methods
Subjects
The study was conducted in the EDs of three metropolitan hospitals in Wellington, New Zealand: Wellington Hospital (tertiary public), Hutt Hospital (secondary public) and Kenepuru Hospital (secondary public). Patients presenting to the ED with asthma were approached by the investigator to assess potential eligibility. Patients aged between 18 and 65 years were eligible for inclusion if they met the following criteria: previous doctor diagnosis of asthma, history consistent with a current acute exacerbation of asthma and a forced expiratory volume in 1 s (FEV1) ≤50% of predicted values at the time of first assessment. Patients with a diagnosis of COPD, or disorders associated with hypercapnic respiratory failure such as neuromuscular disease, chest wall restriction or obesity hypoventilation syndrome, were excluded from the study due to the potential for confounding. Patients who were unconscious, unable to speak or unable to perform spirometry were also excluded. Written informed consent was obtained from each patient.
Study protocol
Patients were randomly assigned to one of two oxygen regimes for 1 h. Patients in the high concentration group received oxygen at a flow rate of 8 l/min via a medium concentration mask (Hudson RCI, Durham, North Carolina, USA) which delivers an Fio2 of between 0.4 and 0.78.25 26 Patients in the titrated group received oxygen only if their saturation was ≤92% on room air, with oxygen titrated as required at 5 min intervals, to achieve an oxygen saturation of 93–95% according to the protocol outlined in the online supplement Table S1. Flow rates up to 4 l/min were delivered via nasal cannulae (Hudson RCI) and those >4 l/min were delivered by medium concentration mask.
A computerised randomisation sequence was generated by the biostatistician (MWe) and patients were enrolled and assigned to their treatment group by the clinical research fellows (MWi, KP, BH, KW and RBo). Allocation concealment was achieved by using a secure database which contained the randomisation sequence. Allocation was only revealed to the researchers when the patients were enrolled and their name entered in the database. Neither investigators nor patients could be blinded to the treatment regimes due to the requirement to titrate oxygen therapy in the control group.
A medical history was taken, each patient underwent a physical examination, and asthma therapy was administered in accordance with published guidelines.22 23 All patients received salbutamol 2.5 mg and ipratropium bromide 0.5 mg via an air-driven nebuliser (Portaneb, Respironics, Murrysville, Pennsylvania, USA) on arrival. Patients with severe asthma (FEV1 30–50% predicted) received salbutamol 2.5 mg via a nebuliser every 20 min and prednisone 40 mg orally. Those with very severe asthma (FEV1 <30% predicted) received salbutamol 2.5 mg via a nebuliser every 15 min, hydrocortisone 200 mg intravenously and magnesium sulfate 2 g in 100 ml of normal saline intravenously over 20 min.
Measures
The transcutaneous partial pressure of carbon dioxide (Ptco2) was used to estimate arterial Paco2 using a combined oxygen saturation/Ptco2 monitor (TOSCA, Radiometer, Basel, Switzerland). Transcutaneous CO2 monitors estimate Paco2 by heating an earlobe probe to 42°C to enhance blood flow and ‘arterialise’ the underlying capillaries. CO2 diffuses through the skin and changes the pH of a thin layer of an electrolyte solution in the probe, and the resulting signal is converted to Paco2. Measurements of Ptco2, FEV1, respiratory rate and heart rate were made at baseline (0 min) and at 20, 40 and 60 min. The oxygen saturation was measured continuously throughout the study period and recorded at 5 min intervals.
Statistical analysis
The prespecified primary outcome variable was the proportion of patients with a Ptco2 >38 mm Hg and FEV1 ≤50% at 60 min. However, after recruitment of the initial 19 subjects it was apparent that the main determinant of this outcome was the baseline Ptco2, rather than whether an increase in Ptco2 had actually occurred. Specifically, of the 3/19 subjects who met the primary end point, two had a decrease in Ptco2 (from 46 to 39 mm Hg and from 45 to 44 mm Hg) and the other had a minimal increase (from 39 to 40 mm Hg). For this reason, after the initial 19 subjects were studied, the primary outcome was changed to the proportion of patients with a Ptco2 rise of ≥4 mm Hg at 60 min, and the proportion of patients with a Ptco2 rise of ≥4 mm Hg and a Ptco2 ≥38 mm Hg at 60 min was included as a secondary outcome variable. Other secondary outcome variables included the mean change in Ptco2 from baseline, changes in respiratory rate, heart rate and FEV1, and the need for hospital admission at the end of the ED treatment period. The proportion of patients with a Ptco2 rise of ≥8 mm Hg was added as a posthoc outcome variable.
The rate of change of Ptco2 was determined using a mixed linear model with random intercept and slope terms.27 In the mixed linear model the fixed effects were the randomised treatment as a dichotomous variable, time as a continuous covariate, and a treatment × time interaction term. A random slope and intercept term with the individual participants as subjects and an unstructured covariance specified for the intercept and slope accounted for the correlation of repeated measurements on the same participants. Continuous outcome variables were analysed as change from baseline using independent sample t tests, or for achieved oxygen saturation for which normality assumptions were not met, by a Mann–Whitney test. Logistic regression was used to model the risk of admission, expressed as an OR, both unadjusted for other variables and adjusted for baseline FEV1, baseline oxygen saturation and baseline Ptco2. Analysis was by intention to treat. SAS version 9.1 and Minitab version 14 were used.
Sample size calculation
Based on previous research19 we calculated that to detect a difference in the proportion of patients with the primary outcome variable of 20% in the high concentration oxygen group and 5% in the titrated group, with power of 80% at a type 1 error rate of 5%, 75 subjects were required in each group.
Results
Eligible patients were recruited from July 2007 to December 2009. A total of 106 patients were randomised, 53 to the high concentration group and 53 to the titrated group. Three patients were withdrawn from the high concentration oxygen group, two due to protocol violations in which the patients met an exclusion criterion after randomisation (one patient with COPD and one with obesity hypoventilation syndrome), and in one patient a reliable Ptco2 signal could not be obtained. As a result there were data from 50 patients in the high concentration group and 53 in the titrated group for final analysis. Figure 1 shows the flow of the patients through the study. The two oxygen treatment groups were well matched with respect to age, sex and respiratory rate (table 1). The mean baseline FEV1 in the high concentration oxygen and titrated oxygen groups was 1.15 and 1.29 litres, respectively.
Flow of patients through the study. ED, Emergency Department; FEV1, forced expiratory volume in 1 s.
Baseline characteristics of patients
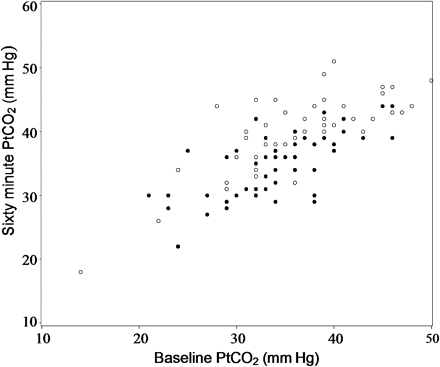
Ptco2 levels at baseline ranged from 14 to 50 mm Hg (figure 2). The majority of patients were hypocapnic at baseline, with 68/103 (66%) having a Ptco2 <38 mm Hg. There were eight patients with an oxygen saturation <93% at baseline while breathing room air. In the titrated oxygen group, 48/53 (90%) patients did not require oxygen therapy throughout the 60 min treatment period, four patients required 1–3 l/min and one required >3 l/min. In the high concentration oxygen group the oxygen saturation at 60 min was ≥99% in 39/50 (78%) patients and was ≥95% in the remaining 11 patients.
The transcutaneous partial pressure of carbon dioxide (Ptco2) levels at baseline and after 60 min in the high concentration (open circles) and titrated (filled circles) oxygen groups. The patient who received high concentration oxygen and was withdrawn after 11 min due to safety concerns, following an increase in the Ptco2 from 41 to 52 mm Hg is not presented in the figure.
The Ptco2 levels at 60 min ranged from 18 to 52 mm Hg (figure 2). One patient who received high concentration oxygen was withdrawn after 11 min due to safety concerns, following an increase in the Ptco2 from 41 to 52 mm Hg. For the categorical outcome variables, the Ptco2 value at 11 min was used as the final measurement in this patient. A total of 10 patients had a final Ptco2 ≥45 mm Hg. All 10 patients were in the high concentration oxygen group, and in five patients there was an increase in Ptco2 ≥10 mm Hg.
The proportion of patients with an increase in Ptco2 of ≥4 mm Hg at 60 min was significantly greater in the high concentration group, compared with the titrated oxygen group, with an RR of 2.3 (95% CI 1.2 to 4.4; p=0.006) (table 2).
The proportion of patients with a predetermined rise in the transcutaneous partial pressure of carbon dioxide (Ptco2) from baseline at 60 min
The proportion of patients with a rise in Ptco2 ≥8 mm Hg was significantly greater in the high concentration group, with an RR of 3.9 (95% CI 1.2 to 13.1, p=0.016). The proportion of patients with a Ptco2 >38 mm Hg and an FEV1 percentage predicted ≤50% after 60 min was 20/49 (40.8%) in the high concentration group and 6/53 (11.3%) in the titrated group, RR 3.6 (95% CI 1.6 to 8.2; p<0.001).
The mean change in Ptco2 from baseline was significantly greater in the high concentration group, with a mean difference between the groups at 60 min of 2.6 mm Hg (95% CI 0.9 to 4.3; p<0.003) (table 3). The proportion of patients with a rise in Ptco2 ≥4 mm Hg was greater in the high concentration group at the 20 and 40 min time points (table 3). The rate of increase in the high concentration group was 0.054 (95% CI 0.035 to 0.074) mm Hg/min and in the titrated group it was 0.012 (95% CI −0.0065 to 0.031) mm Hg/min. The difference in the rate of change was 0.042 mm Hg/min (95% CI 0.069 to 0.15, p=0.003).
Time course of changes in the transcutaneous partial pressure of carbon dioxide (Ptco2) in the treatment groups
There were 26/50 (52%) of the high concentration group admitted to hospital compared with 17/53 (32%) in the titrated group, OR 2.29 (95% CI 1.03 to 5.10, p=0.042). After adjusting for baseline FEV1, baseline oxygen saturation and baseline Ptco2, this OR was 1.7 (95% CI 0.68 to 4.26, p=0.257) (table 4). In the adjusted analysis, a higher baseline FEV1 and oxygen saturation were associated with a reduced risk of admission. There was no difference between the treatment groups in the mean change of respiratory rate, pulse rate or FEV1 over 60 min (see online supplement Table S2).
Risk of hospital admission
Discussion
This randomised controlled trial has shown that high concentration oxygen therapy results in a significant increase in Ptco2 compared with titrated oxygen when administered to patients presenting to the ED with severe exacerbations of asthma. We propose that the results are of physiological and clinical significance, as indicated by the two- to fourfold RR of an increase in Ptco2 of at least 4 or 8 mm Hg, respectively, in the group receiving high concentration oxygen. Furthermore, all 10 patients who developed hypercapnia with a final Ptco2 ≥45 mm Hg had received high concentration oxygen therapy. This observation suggests that the administration of high concentration oxygen in the ED setting is a determinant of the development of respiratory failure, a recognised marker of near fatal asthma.22 After adjustment for baseline predictors of severity, high flow oxygen was not statistically significant as a predictor of hospital admission, although the point estimate was consistent with an increased risk.
The results of the current study extend those of the only previous randomised controlled trial of high concentration oxygen in acute severe asthma.19 In this previous study, 74 patients were given 100% or 28% oxygen for 20 min on arrival to the ED prior to receiving any asthma treatment. The difference in the mean rise in Paco2 between the groups was 2.7 mm Hg, similar to the 2.6 mm Hg noted in the current study. However, the duration of oxygen therapy was only 20 min and no concurrent asthma treatment was administered, limiting the generalisability of their study findings. Also, the administration of 28% oxygen rather than a titrated oxygen regime differed from the therapeutic approach currently recommended in guidelines.22–24 Our study and others8 have shown that most adult patients presenting to the ED with severe exacerbations of asthma do not have hypoxaemia, and, as a result, do not require initial oxygen therapy.
There are a number of methodological issues relevant to the interpretation of the study findings. The first is that we used a transcutaneous CO2 monitor to measure Paco2 rather than the ‘gold standard’ arterial blood gas (ABG) test. This method was chosen as it allowed continuous Ptco2 monitoring without the discomfort of multiple ABG sampling or the risk of hand ischaemia associated with indwelling radial artery cannulae. The accuracy of transcutaneous CO2 monitoring has been demonstrated in a variety of settings including healthy subjects,28 AECOPD,29 sleep disorders,30 critical illness31 and in a mixed group of patients presenting to an ED.32 We also assessed the accuracy of our device in a subset of patients who had simultaneous ABG and Ptco2 recordings.33 It accurately measured Paco2 without significant bias and with clinically acceptable limits of agreement when compared with the ABG measurement, thus validating our methodology.
By necessity the study was unblinded, as there was a clinical requirement for the investigator to have knowledge of the oxygen saturations in order to titrate the oxygen therapy in the ‘control’ treatment group. The objective display of Ptco2 on the monitor avoided subjective assessment of the primary outcome variable. In the titrated oxygen group, the target oxygen saturation was 93–95% to ensure both that hypoxaemia was relieved and that hyperoxaemia was avoided.
Our prespecified analysis plan was to use the proportion of patients with a Ptco2 >38 mm Hg and FEV1 ≤50% at 60 min as the primary outcome variable. However, in the early phase of recruitment it was apparent that the prespecified primary outcome variable was primarily determined by the presenting Ptco2 rather than by a physiological increase in Ptco2. After a review of the records of the first 19 patients (representing 17% of 106 patients contributing to the main outcome analysis) we registered a change in the primary outcome variable to the proportion of patients with a Ptco2 rise of ≥4 mm Hg. We acknowledge that changing the primary outcome variable after the start of the study raises the possibility of creating a biased assessment of the outcome of the trial. However, no formal interim statistical analysis, of either the prespecified outcome variable or the new main outcome variable, was carried out prior to this decision and, although the study itself was not masked as to treatment allocation, the decision was made without reference to the randomised allocation of the patients. In the event, for the prespecified main outcome variable, Ptco2 >38 mm Hg and an FEV1 percentage predicted ≤50% after 60 min, the 3.6-fold increased risk associated with high concentration oxygen therapy was similar to the 2.3- and 3.9-fold increased risk observed with a Ptco2 rise of ≥4 and ≥8 mm Hg, respectively.
We had intended to recruit 150 patients, based on the power calculation derived from the previous randomised controlled trial of oxygen therapy in asthma.19 Due to difficulties with recruitment, we extended the study sites to include Hutt Hospital, and the planned 2 year study period by 6 months. With these measures we enrolled 106 patients, which provided sufficient statistical power to determine clinically relevant differences between the treatment groups. Patients with a diagnosis of COPD were excluded due to the known effect of high concentration oxygen in exacerbations of this disorder.1–7 It is probable that greater increases in Ptco2 may occur in an unselected population of patients with acute asthma, which is more likely to include those with concomitant undiagnosed COPD or other disorders associated with chronic respiratory failure.
Although an attempt was made to include potential patients with severe or life-threatening asthma, patients who were moribund, unable to speak, unable to perform spirometry or so distressed that they could not consent were not enrolled. Consequently, those with the most severe airflow obstruction, and hence the highest risk of hypercapnia at presentation, were not able to be studied. In this regard it is relevant that with progressively more severe hypercapnia, smaller falls in alveolar ventilation are required to produce a given further rise in Paco2.34
The increase in Paco2 with high concentration oxygen demonstrated in this study is also likely to be an underestimate of the magnitude of the effect that may be seen in standard clinical practice, in which oxygen therapy may be administered for a longer period. The Ptco2 progressively increased in the high concentration group throughout the 60 min study period, suggesting that some patients may have had further increases in Ptco2 had the high concentration oxygen regime continued.
The main mechanism for the elevation in Ptco2 demonstrated in this study is likely to be worsening ventilation–perfusion mismatching as a result of the release of hypoxic pulmonary vasoconstriction and a consequent increase in physiological dead space. This has been demonstrated in studies of the effects of oxygen therapy in both acute severe and chronic asthma,10–15 and is one of the main mechanisms which causes oxygen-induced CO2 retention in AECOPD.3–7 As a result, one of the important clinical implications of our study is that high concentration oxygen therapy may have the potential to cause an increase in Paco2 across a range of respiratory conditions with abnormal gas exchange due to ventilation–perfusion inequality. In support of this interpretation, this physiological response to high concentration oxygen therapy has now been reported in stable COPD,5 7 AECOPD,1 2 4 6 asthma,10–12 18 19 21 obesity hypoventilation syndrome35 36 and diffuse pulmonary fibrosis or infiltration.37 This response contrasts with that observed in normal subjects in whom high concentration oxygen therapy leads to a small decrease in Paco2.38 39 The observation that there was no difference in the change in FEV1 between the two regimes suggests that the increase in Ptco2 with high concentration oxygen therapy was not due to a bronchoconstrictor effect related to the low humidity of the delivered oxygen.
We conclude that high concentration oxygen increases the risk of hypercapnia in patients with severe exacerbations of asthma. Our findings also suggest that the potential increase in Paco2 with high concentration oxygen therapy is not limited to asthma and COPD, but may also occur in other respiratory disorders with abnormal gas exchange. Consistent with recent guidelines,22–24 we recommend a titrated oxygen regime in patients with severe exacerbations of asthma, in which oxygen is administered only to those with evidence of arterial hypoxaemia, in a dose that relieves the hypoxaemia without causing hyperoxaemia, thereby obtaining the benefits while reducing the potential for harm.
References
Supplementary materials
Web Only Data thx.2010.155259
Files in this Data Supplement:
Web Only Data thx.2010.155259
Files in this Data Supplement:
Footnotes
See Editorial, p 931
Linked article 161497.
Funding Funding was received from the Health Research Council of New Zealand, the Wellington Hospitals and Health Foundation, the Asthma and Respiratory Foundation of New Zealand and the Royal Australasian College of Physicians.
Competing interests None.
Ethics approval This study was conducted with the approval of the Central Regional Ethics Committee.
Provenance and peer review Not commissioned; externally peer reviewed.
Linked Articles
- Editorial
- Airwaves