Abstract
Optimizing patient-ventilator synchrony is essential in managing patients who require prolonged mechanical ventilation in the long-term acute-care hospital. Inadequate synchrony can increase work of breathing, cause patient discomfort, and delay both weaning and general rehabilitation. Achieving optimal synchrony in the long-term acute-care hospital depends on a number of factors, including adjusting ventilator settings in response to improving lung function; adjusting psychotropic medications to control delirium, anxiety, and depression; and ensuring there is a well positioned correctly sized tracheostomy tube in the airway. The purpose of this review is to provide an update on issues pertinent to patient-ventilator synchrony in the LTACH setting.
Introduction
In the United States an increasing number of patients are surviving critical illness, failing to wean quickly from mechanical ventilation, and are being transferred to long-term acute-care hospitals (LTACHs) for weaning from prolonged mechanical ventilation.1–4 The goals of mechanical ventilation in the LTACH are no different from those of the intensive care unit (ICU), and focus on the provision of adequate gas exchange while unloading the respiratory muscle pump until the cause of the respiratory failure is reversed.
Optimal patient-ventilator synchrony is essential for efficient mechanical ventilation and patient comfort, and allows for a decrease in the work of breathing. Ensuring optimal patient-ventilator synchrony may help reduce ventilator-induced lung injury and ventilator-induced diaphragmatic dysfunction, both of which may further prolong the episode of mechanical ventilation. This paper reviews patient-ventilator asynchrony in the LTACH setting.
Overview of Prolonged Mechanical Ventilation at the Long-Term Acute-Care Hospital
Prolonged mechanical ventilation is usually defined as the need for mechanical ventilation for longer than 21 days.5 The placement of a tracheostomy tube (usually following 7–14 days of mechanical ventilation with an endotracheal tube), can also be considered the point in time when mechanical ventilation becomes prolonged. These patients represent a select population of patients who fail multiple attempts at weaning, either in the ICU or in a step-down unit.
The pathophysiology of weaning failure has been demonstrated6 to be due to a reduction in respiratory muscle strength, abnormal lung mechanics, and increased respiratory drive associated with an inability to generate adequate tidal volumes during spontaneous breathing trials. Reduced respiratory muscle strength and an increase in lung impedance result in an increase in the ratio of respiratory load to capacity. Until these problems resolve, attempts at spontaneous breathing are destined to fail.6 Mechanical ventilation is continued until sufficient recovery of the respiratory system allows liberation from mechanical ventilation, is continued indefinitely to maintain pulmonary function in those patients unable to wean, or is electively withdrawn in those patients who do not wish to live dependent on mechanical ventilation.7
Optimizing patient-ventilator synchrony in the LTACH may shorten weaning time and improve sleep quality.8 Whether optimizing patient-ventilator synchrony also improves outcomes, reduces complications, improves sleep quality,8 or reduces the cost of care is unknown at this time. Asynchrony may be a marker of severity of illness rather than a factor that directly affects outcome.
Over the past 15 years the LTACH has become an important destination for patients who require prolonged mechanical ventilation, with about 10% of all patients at LTACHs receiving mechanical ventilation. Table 1 shows the types of ventilators available in selected LTACHs in the United States. Other locations where patients receive prolonged mechanical ventilation include step-down units in acute-care hospitals, skilled nursing facilities, and home.9
Ventilator Models in Use in Selected Long-Term Acute-Care Hospitals in the United States
A multicenter prospective study3,4 described the clinical characteristics of patients admitted to LTACHs for prolonged mechanical ventilation. The pulmonary diagnoses in the cohort included: COPD (42%), obstructive sleep apnea/obesity hypoventilation (8.2%), interstitial lung disease (2.7%), post-lung-resection (2.7%), pulmonary vascular disease, and bronchiectasis. In the LTACH setting, patients receive long-term acute medical care while undergoing multidisciplinary rehabilitation to optimize function. Data from multiple studies show that about 50% of patients undergoing prolonged mechanical ventilation are eventually weaned from mechanical ventilation,2,4,10 but a substantial percentage of these patients cannot be subsequently decannulated.11 The one-year survival rate remains disappointingly low in patients weaned from prolonged mechanical ventilation.1
Patient-Ventilator Synchrony in the LTACH Setting
In order to optimize patient-ventilator synchrony, the settings on the mechanical ventilator must approximate the patient's physiological needs. In a synchronous patient-ventilator system, every breath initiated by the patient is matched by the ventilator during the respiratory cycle. Patient-ventilator asynchrony is usually detected in studies using esophageal pressure monitoring to determine respiratory effort. However, the esophageal balloon is not in widespread clinical use, and some studies have suggested that asynchrony can also be accurately detected less invasively using graphic displays of flow and airway pressure signals.6,12 Others have suggested that accessory muscle use can be used to detect trigger asynchrony.13
Patient-ventilator asynchrony is categorized according to when it occurs in the 4 phases of the respiratory cycle: trigger, inspiration, cycle, or expiration. Incorrectly setting the triggering sensitivity setting can cause auto-triggering, excessive triggering delay, or ineffective respiratory efforts. Patients undergoing prolonged mechanical ventilation in the LTACH via a tracheostomy tube can develop auto-triggering in the presence of a tracheostomy cuff leak, during speech, swallowing or chewing, or with patient movement during physical therapy. Some triggering delay is probably unavoidable, as ventilator breaths are not triggered until the machine detects flow or pressure changes.14 However, excessive triggering delay can significantly increase the work of breathing.15 A novel mode of mechanical ventilation called neurally adjusted ventilatory assist (NAVA)16 can eliminate triggering delay. In NAVA the ventilator is triggered by electrical activity of the diaphragm. This optimizes synchrony between mechanical inspiration and neural respiratory drive and eliminates the problems of respiratory drive due to intrinsic PEEP and impaired respiratory drive.
Patients with COPD represent about 40% of patients undergoing prolonged mechanical ventilation in the LTACH.3,4 The pressure-control continuous mandatory ventilation (PC-CMV) mode is commonly used to transition this patient population to weaning from the mechanical ventilator. Failure to trigger the ventilator despite patient effort is common in patients with COPD, due to dynamic hyperinflation and intrinsic PEEP.6 Triggering time is further increased with increasing amount of pressure support.17 This is due to inspiratory activity beginning before elastic recoil pressure has returned to a level that can be overcome by the patient's respiratory muscle effort18 and is usually seen with intrinsic PEEP. Others have shown that ineffective triggering can be reduced by reducing pressure support and inspiratory time.13,19 A detailed review of this topic, along with graphics, is available.17 Adjusting PEEP appears to have no effect on ineffective triggering in the LTACH setting,13 but it is worth noting that, in general, increasing extrinsic PEEP does improve trigger asynchrony in the setting of increased intrinsic PEEP. Others have demonstrated a U-shaped relationship between the amount of pressure support provided and patient comfort, as measured using the Borg scale and a visual analog scale. Therefore, increasing pressure support is not necessarily associated with increased patient comfort.20 While many studies have suggested that adjusting external PEEP may help reduce ineffective triggering,14 the study by Chao et al did not show a consistent effect of adjusting PEEP on ineffective triggering.13 Furthermore, intrinsic PEEP may vary breath by breath, and that in turn complicates the ability to pick an “optimal” extrinsic PEEP that can consistently decrease the work of breathing.
During the inspiratory phase of mechanical ventilation, a mismatch between the ventilatory requirements of the patient and the delivered machine breath can lead to inadequate or excessive assistance from the mechanical ventilator. Inadequate ventilator assistance leads to insufficient unloading of the respiratory muscles. On the other hand, excessive ventilator assistance increases the risk of worsening dynamic hyperinflation and intrinsic PEEP in patients with obstructive disease. Excessive assistance can also cause hypocapnia and episodes of central apneas, which in turn may increase sleep arousals during mechanical ventilation.12
Setting the flow rate either too high or too low can lead to an increase in the work of breathing. Breaths may be terminated prematurely, leading to double-triggering (flow rate too high), or terminated late, leading to patient discomfort and an inadequate expiratory time due to prolonged inspiratory time. Late termination of inspiration has been shown to exacerbate dynamic hyperinflation in patients, due to an inability of patients to compensate fully for a reduction in expiratory time.21 Cardiac oscillation, which can be seen on the pressure tracing on the monitor, has been reported to cause auto-triggering, which may result in hyperinflation in either assist control or pressure support ventilation.22 Cardiogenic oscillation and auto-triggering may interfere with weaning by overestimating tidal volume generated during pressure-support weaning.
The ventilation mode can also affect patient-ventilator synchrony. In the LTACH setting patients can usually be ventilated using either volume control continuous mandatory ventilation (VC-CMV) or PC-CMV. Patients with end-stage restrictive lung disease, such as pulmonary fibrosis, who choose to undergo prolonged mechanical ventilation may require a pressure-control mode to avoid barotrauma. Patient-ventilator asynchrony and hyperventilation occur in both VC-CMV and pressure-control modes.19 Yang et al compared PC-CMV and VC-CMV in patients receiving low-tidal-volume ventilation, and found that the pressure-targeted approach was associated with less inspiratory effort during the triggering phase but not the flow-delivery or the cycle phase of the assisted breath.23 The work of breathing and amount of asynchrony did not appear to differ between the 2 modes. Air-trapping was more severe during VC-CMV, as compared with PC-CMV. Parthasarathy et al showed that central apneas occur at night with PC-CMV, but not with VC-CMV, resulting in increased risk of sleep fragmentation during pressure support ventilation.8
Proportional assist ventilation reduces patient-ventilator asynchrony, as compared with PC-CMV.24 Asynchrony was significantly correlated with the number of arousals per hour (R2 = 0.65). Multivariate analysis showed that overall sleep quality (but not sleep quantity) was significantly improved with proportional assist ventilation. Others have had similar findings in patients randomized to either PC-CMV or proportional assist ventilation, with patients on proportional assist ventilation more likely to remain on a spontaneous breathing mode and less likely to have patient-ventilator asynchrony.25 Compared with PC-CMV, NAVA limited the risk of over-assistance, avoided patient-ventilator asynchrony, and improved overall patient-ventilator interaction.16
Sleep quality appears to be important in recovery from critical illness. Sleep has been shown to be adversely affected by mechanical ventilation.26 Optimizing patient-ventilator synchrony would be expected to enhance sleep quality. In a study that compared sleep quality in patients receiving either clinically or automatically adjusted PC-CMV (some via tracheostomy) or VC-CMV, no difference in ineffective respiratory efforts or sleep apnea was observed.27 Patients had on average 7–16 ineffective respiratory efforts and 5–7 central apneas per hour of sleep. Others have found an increase in central sleep apnea during PC-CMV, as compared with VC-CMV, but this finding may have been due to over-assistance with ventilatory needs during sleep.18
As the patient in the LTACH recovers from critical illness, the number of hours of mechanical ventilation required by the patient in a 24-period should be adjusted. Daily attempts at weaning are made using tracheostomy mask protocols or reductions in PC-CMV. The initial goal of these weaning attempts is to liberate the patient from the mechanical ventilator for a period of time during the day, which allows for greater participation in physical therapy. Portable ventilators can be used for ambulation in patients unable to come off the mechanical ventilator. These smaller ventilators, such as the Newport, may not have a graphics package, thus limiting assessment of synchrony.
Risk Factors for Patient-Ventilator Asynchrony in the LTACH
Some data are available on patient-ventilator synchrony in the LTACH setting. In a study of 174 consecutive patients undergoing prolonged mechanical ventilation in a regional weaning center, trigger asynchrony was detected in 11% of patients.13 Those with trigger asynchrony were more likely to have COPD and were less likely to wean from mechanical ventilation, despite attempts at limiting asynchrony. Interestingly, decreasing the pressure support eliminated asynchrony, at the cost of causing shallow breathing and respiratory distress. Those patients with trigger asynchrony had a longer median time to wean from mechanical ventilation (72 days vs 33 days). Similarly, asynchrony prolongs mechanical ventilation in the ICU and contributes to prolonged mechanical ventilation. Thille et al studied 62 consecutive patients requiring mechanical ventilation for more than 24 hours (not all subjects met the criteria for prolonged mechanical ventilation) and found that ineffective triggering and double-triggering constituted 98% of asynchronous breaths.28 Patients on assist-control ventilation had both significantly longer duration of mechanical ventilation and higher mortality rate than patients on pressure support. Double-triggering was more common during assist-control than during pressure support. Patients who had an asynchrony recorded in greater than 10% of respiratory efforts had significantly longer mechanical ventilation and were more than 8 times as likely to undergo tracheotomy.
When a patient is transferred to the LTACH from the ICU, it is important to review the ventilator settings, as they may not have been readjusted in the ICU as the patient's lung pathophysiology improved. Maintaining low-tidal-volume settings (6 mL/kg) despite improving lung compliance as acute lung injury or acute respiratory distress syndrome resolves can result in atelectasis, reduced lung compliance, and widening of the alveolar-arterial oxygen difference,29 so patient-ventilator synchrony may not be optimized. Daily ventilator checks (that include calculation of the lung compliance) and the availability of pressure-volume graphs can help ensure the correct settings.
The hemoglobin level that optimizes weaning from prolonged mechanical ventilation is not known. We recently found that patients who successfully wean from prolonged mechanical ventilation have a hemoglobin of 11 g/dL, indicating that target hemoglobin level used in the ICU may be too low in the LTACH setting.30 This may reflect the increased need for oxygen carrying capacity as the patient mobilizes during the weaning process. The minimal threshold of hemoglobin needed to wean from prolonged mechanical ventilation is still not known.
Over-sedation and delirium are both risk factors for patient-ventilator asynchrony, ineffective triggering, and failure to wean from mechanical ventilation in the ICU. Greater sedation is associated with decreased respiratory drive and ineffective triggering.31 In the LTACH setting, delirium may be a greater contributor to patient-ventilator asynchrony, as sedation is usually minimized to facilitate weaning and participation in therapy. Generalized anxiety and depression can also interfere with patient-ventilator synchrony in the LTACH setting. Jubran et al prospectively evaluated 478 patients admitted to an LTACH for prolonged mechanical ventilation via tracheostomy for the presence of depressive disorders.32 On admission, delirium was diagnosed in 246 patients (52%) and coma in 48 patients (10%). They identified transient and persistent types of delirium. Persistent delirium was associated with a neurological cause of respiratory failure. Risk factors for any type of delirium included greater age, elevated Acute Physiology and Chronic Health Evaluation (APACHE) score, and a higher comorbidity index. LTACH stay was significantly longer in the delirium group (35 days), as compared to those without delirium (31 days). A depressive disorder was diagnosed in 142 patients (42%) of the cohort and was associated with a higher APACHE score, a higher comorbidity index, and longer duration of mechanical ventilation prior to transfer to the LTACH. Patients with depressive disorders were 3 times more likely to fail weaning attempts. Jubran et al speculated that failure to wean may have been linked to depression causing an inability to psychologically tolerate spontaneous breathing trials. Specifically, anxiety-related tachypnea can interfere with the interpretation of a spontaneous breathing trial. Despite controlling for age and comorbidity, mortality was 2.4-fold higher among the patients with depressive disorders. Other factors that can interfere with patient-ventilator synchrony include critical-illness neuromyopathy, with reduced respiratory muscle pump function causing trigger asynchrony.
Other factors associated with patient-ventilator asynchrony have been identified in patients undergoing short-term as opposed to prolonged mechanical ventilation. Thille et al found that low PaO2/FIO2, volume assist control ventilation, short inspiratory time, high maximum inspiratory pressure, high PEEP, male sex, elevated bicarbonate, and alkalosis all were associated with double-triggering.28 Poor trigger sensitivity, high tidal volume, high peak inspiratory pressure, and high pressure support were associated with ineffective triggering, whereas severity of illness was not associated with a high incidence of ineffective triggering.
Detecting Patient-Ventilator Asynchrony in the LTACH Setting Without Benefit of Graphics
A ventilator equipped with a graphic display may not be available to every patient in the LTACH setting (see Table 1). However, asynchrony can be readily identified by carefully monitoring patient-ventilator interaction at the bedside, even without the benefit of a graphic display. Patient-ventilator asynchrony in the non-sedated patient can cause increased work of breathing, which can cause physical signs, including anxiety, agitation, tachypnea, tachycardia, accessory muscle recruitment, nasal flaring, tracheal tugging, and paradoxical breathing.33 If the patient has a tracheostomy tube in position, asynchrony may cause the patient to breath with an open mouth. Patients with substantial asynchrony are often noted by caregivers to be “bucking” or “fighting” the ventilator.
In some patients the signs of patient-ventilator asynchrony can be much less obvious and may be missed by the untrained observer. Chao et al highlighted accessory muscle use at the bedside as a way to identify patient-ventilator asynchrony, and noted uncoupling of respiratory effort from ventilator triggering (a sign of asynchrony) in about 10% of patients.13 Tobin et al quantitatively assessed asynchrony in 17 patients undergoing spontaneous breathing trials and found that patients who failed a spontaneous breathing trial displayed significantly greater asynchrony and paradoxical movement of the rib cage and abdomen than those who succeeded spontaneous breathing trails.34 Abdominal muscle activity during expiration and ventilator-breath delivery prior to complete exhalation are additional subtle signs of asynchrony.
The alarms on a ventilator without graphics can also be a useful indicator of asynchrony. For example, asynchrony can lead to early breath-termination, which may lead to a respiratory rate and tidal volume outside of a set range.
These bedside observations can help practitioners detect patient-ventilator asynchrony and have value even when a graphics package is available.
Role of the Tracheostomy Tubein Patient-Ventilator Synchrony
The placement of a tracheostomy tube is considered by some to mark the transition from acute to prolonged mechanical ventilation. Studies have shown that the tracheostomy tube may help the weaning process by reducing the work of breathing and intrinsic PEEP, and may reduce ineffective triggering by improving expiratory flow, which reduces intrinsic PEEP.35 The mechanism underlying these effects is not well understood, but may relate to the shorter length and more rigid form of the tracheostomy tube, as compared to an endotracheal tube. The tracheostomy tube allows for improved oral hygiene and may reduce aspiration risk during swallowing. Tracheostomy may improve patient comfort, reduce the need for sedation, enhance secretion removal, shorten the time to patient mobilization, and enhance patient communication, and it allows for flexibility in stopping and starting ventilator support during tracheostomy mask trials. It is not known if tracheostomy per se shortens weaning time, compared with continued attempts at weaning via an endotracheal tube. Some data indicate that earlier percutaneous tracheostomy (within 48 hours, as compared with after 14 days) may reduce ICU stay and time on the mechanical ventilator,36 but that view is controversial, and other studies have found no difference between early tracheostomy and prolonged intubation.37
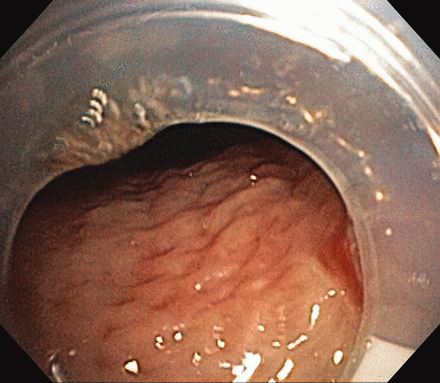
The tracheostomy tube is likely to be a source of patient-ventilator asynchrony that may interfere with weaning and subsequent recovery, although data supporting this theory are not available, to our knowledge. Tracheostomy tube malposition can be detected in approximately 10% of patients with prolonged respiratory failure.38 To function well, the tracheostomy tube needs to be centered in the airway, above the carina. A malpositioned tube (Fig. 1) or one that is blocked with granulation tissue (Fig. 2) can increase the peak inspiratory pressure, with patient-ventilator asynchrony, increased oxygen requirement, and failure to tolerate weaning to either pressure support or tracheostomy mask.11,38 (See recent review articles for further details on tracheostomy management.39,40) Selecting an appropriate-size tracheostomy tube and centering it in the airway is essential to optimizing patient-ventilator synchrony. If the tracheostomy tube is the wrong size or the curvature incorrectly fits the tracheal anatomy, the tube may not be centered in the airway, which in turn may substantially affect air flow and triggering, which may increase asynchrony and prolong mechanical ventilation.40 A too-short tube may abut the posterior tracheal wall and eventually form a tracheoesophageal fistula. A too-long tube may be occluded by either the anterior tracheal wall or the carina. The innominate artery is adjacent to the anterior wall of the trachea, and pressure in that area can create a tracheo-innominate-artery fistula, which can result in fatal bleeding into the airway. The airway can be compromised by other factors, such as the development of tracheal stenosis, tracheomalacia, or granulation tissue formation. A longer tube may be necessary to bypass such areas of airway obstruction. It is important to have a low threshold to perform endoscopy, to ensure the tracheostomy tube is correctly positioned in patients who develop patient-ventilator asynchrony or failure to wean.38
Tracheostomy tube partially occluded by the posterior tracheal wall, as a result of malposition within the airway.
Tracheostomy tube partially occluded by granulation tissue in a patient with osteogenesis imperfecta.
The accumulation of dried secretions in the endotracheal or tracheostomy tube lumen reduces the inner diameter and markedly increases airway resistance (air flow through the tube is related to the fourth power of the tube radius). This can be a particular problem with tracheostomy tubes that may remain in place for up to 2–3 months, depending on the manufacturer's recommendations.40
Summary
Patient-ventilator synchrony constitutes an important cornerstone in management of patients with prolonged mechanical ventilation in the LTACH setting. Asynchrony is associated with prolonged mechanical ventilation and probably contributes to sleep disruption and delirium. Many factors contribute to asynchrony, including the tracheostomy tube, the ventilator, and the patient. Effective strategies to improve patient-ventilator interaction in the LTACH include adjusting ventilator settings, selecting an appropriate-size tracheostomy tube, and the judicious use of psychotropic medications. In selected cases, the use of innovative ventilation modes may be considered. Minimizing patient-ventilator asynchrony may contribute to decreasing the duration of mechanical ventilation, minimizing complications, and decreasing patient mortality.
Footnotes
- Correspondence: Alexander C White MD, Department of Pulmonary and Sleep Medicine, Rose Kalman Research Center, New England Sinai Hospital, 150 York Street, Stoughton MA 02072. E-mail: awhite{at}nesinai.org.
-
Dr White has disclosed a relationship with Breath Technology. Dr Ghamloush and Dr O'Connor have disclosed no conflicts of interest.
-
No discussion follows this paper; Dr White was unable to attend the conference.
- Copyright © 2011 by Daedalus Enterprises Inc.