Introduction
Asthma is a complex disease of airway inflammation, which results in airway remodeling and hyper-responsiveness.1 Because asthma-related symptoms are not specific to asthma, diagnosis requires objective data from pulmonary function tests (PFT). Bronchodilator responsiveness and airway responsiveness to provocational stimuli (eg, methacholine, dry-gas hyperpnea) help establish the diagnosis of asthma.1 This report describes a patient who had several asthma risk factors and marked responsiveness to inhaled bronchodilators, but no airway responsiveness to methacholine inhalation.
Case Summary
A 58-year-old white man presented to our PFT laboratory for a methacholine challenge test because of dyspnea on exertion, especially when climbing the stairs at his place of employment, a paper mill, which he characterized as “very dusty.” He reported a personal history of atopy and that his brother was asthmatic. He quit smoking at age 42, after 20 pack-years. His body mass index was 27.5 kg/m2. He reported symptomatic improvement following 6 months of treatment with a formoterol/budesonide inhaler, which he had stopped using 48 hours prior to the PFTs. He denied exercising on the day of testing.
Table 1 shows the baseline spirometry, whole-body plethysmography, and diffusing-capacity results. All the tests satisfied the American Thoracic Society criteria for acceptability and reproducibility. Comparison to the results from spirometry without bronchodilator, performed 17 months earlier, revealed that his baseline forced vital capacity and FEV1 had declined by 330 mL (−7.7%) and 430 mL (−12.5%), respectively. A methacholine challenge test was performed with the 5-breath dosimeter technique2 and nebulized methacholine (Provocholine, Methapharm, Brantford, Ontario, Canada, delivered with a 646 nebulizer, DeVilbiss, Healthcare, Somerset, Pennsylvania). The provocational concentration of methacholine that caused a 20% decrease in FEV1 (PC20) was > 20 mg/mL. Immediately following the methacholine challenge test, 2.5 mg albuterol and 0.5 mg ipratropium were administered via nebulizer, and 20 min later spirometry was repeated (Table 2).
Baseline Pulmonary Function Test Results
Methacholine Challenge Test Results
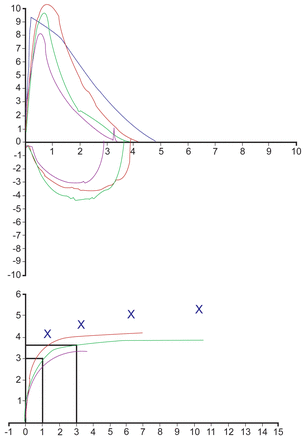
Figure 1 shows the flow-volume loops and volume-time curves from baseline spirometry, post-methacholine, and post-bronchodilator testing. Despite the lack of airway responsiveness to methacholine, he had a substantial response to inhaled bronchodilator: FEV1 increased by 800 mL (30%) above the methacholine-challenge-test nadir, and by 480 mL (16%) above the baseline spirometry. The post-bronchodilator FEV1 was nearly identical to the baseline FEV1 17 months prior (within 0.05 mL) (Table 3).
Flow-volume loops and volume-time curves. from baseline spirometry (green), after methacholine (violet), and after bronchodilator (red).
Changes in FEV1
Discussion
This patient presented with several risk factors for asthma, including atopy, genetic predisposition, variable FEV1, and dyspnea on exertion, which seemed to be intensified by a dusty work environment. With so many risk factors I would appraise the pre-methacholine-challenge-test probability of asthma as greater than 50%. In addition, the PFT data showed a substantial bronchodilator response after methacholine challenge test, which adds an additional asthma risk factor. So why didn't this patient demonstrate substantial airway responsiveness to methacholine? There are 3 scenarios to consider as explanations for this patient's PFT data.
Scenario 1: Reversible Restriction
This patient may not have asthma at all, and his bronchodilator response was an example of “reversible restriction,” which is a restrictive pattern on spirometry, reduced lung compliance, and reduced lung volumes that improve following bronchodilator inhalation.3–5 The mechanism of reversible restriction is closure of alveolar ducts and terminal bronchioles by airway-smooth-muscle constriction, which results in loss of functional parenchyma.3 In one reported case, Kaminsky and Irvin3 found no evidence of airway-smooth-muscle proliferation and hypothesized that muscle function (and not added bulk) was responsible for airway closure. In addition to airway-smooth-muscle constriction, the presence of fibrin in terminal airways inactivates local surfactant, resulting in elevated airway surface tension and ultimately airway closure. In addition to airway-smooth-muscle relaxation, β-agonist-induced increases in cyclic adenosine monophosphate stimulate the secretion of surfactant from alveolar type II cells, via protein kinase A activation, potentially resulting in recruitment and stabilization of distal airways.5 Reversible restriction has been reported in patients with asthma, bronchiolitis,3 hypersensitivity pneumonitis,6 and reactive airway disease syndrome.7 Despite borderline restriction on baseline spirometry, reversible restriction is unlikely in this patient, given his normal baseline lung volumes and diffusing capacity.
Scenario 2: False Negative Due to Technical or Procedural Factors
There are numerous technical and procedural factors that can spuriously reduce the airway response to methacholine and cause a false-negative test.2 First, the methacholine powder must be mixed correctly with normal saline, with or without 0.4% phenol (preservative), under sterile conditions. Methacholine should be stored at about 4°C, but should be allowed to warm to room temperature before administration. Provocholine can be stored for up to 5 months without loss of potency. There is no evidence that the limited response to the methacholine challenge test in this patient was due to diminished methacholine potency. Our laboratory follows the recommendations for handling and storage, and we label the methacholine vials with the expiration date.
Another important factor in methacholine administration is nebulizer performance. The suggested nebulizer output is 0.009 mL ± 10% from a 0.6-second dosimeter-controlled nebulization time.2 Factors that affect the performance of the DeVilbiss 646 nebulizer include the gas flow, the opening or closing of the nebulizer air vent (open position increases output), the position of the impinger arm, and the distance between the capillary tube and the jet orifice. While I do not believe that poor nebulizer performance was responsible for this patient's limited response to methacholine, I cannot state this definitively, because I did not measure that nebulizer's output.
The patient's inspiratory flow, volume, and breath-hold time can also affect methacholine deposition. These variables can be difficult to control and should be explained and demonstrated to the patient before starting the methacholine challenge test.
Scenario 3: False Negative Due to Bronchoprotective Effects of Deep Inspirations and Corticosteroids
Another possibility is that the patient may indeed have asthma, but the bronchoprotective effect of deep inspirations8–12 required by the dosimeter technique2 to deliver methacholine and treatment with corticosteroids produced a false-negative result. It is well established that the act of breathing has functions beyond gas exchange, one of which is modulation of airway-smooth-muscle tone.8,9 In health, airway-smooth-muscle is prevented from realizing its maximum force-generating capacity because of the mechanical stretch imposed by breathing8,9 and the surrounding parenchyma.13 The mechanical stretch from tidal breathing and deep inspirations disrupt and limit actin-myosin binding in airway-smooth-muscle cells, which keeps the airways in a relaxed or “melted” state.8,9 Moreover, the cytoskeleton of airway-smooth-muscle cells behaves like a “soft glassy material,”8 which is a soft solid that fluidizes when shear stress is applied and returns to solid after the stress is removed.8,14,15 A classic example of an innately soft glassy material is ketchup.8 If one observes ketchup in a bottle that has been undisturbed, the ketchup will appear thick and will resist flowing out of the bottle. However, if one vigorously shakes the ketchup bottle for a long enough period of time, the ketchup fluidizes and flows more easily. Allow the bottle to stand undisturbed and it will return to a more solid or “frozen” state. As a soft glassy material, the cytoskeleton of the airway-smooth-muscle cell is kept in a more relaxed, melted, fluid state by the shear stress produced by tidal breathing and deep inspirations.
The effect of breathing on airway-smooth-muscle tone is very powerful. In a study of maximally constricted bovine airway-smooth-muscle, muscle stiffness was reduced by 50% after a less than 3% stretch amplitude was applied.16 When deep inspirations are prohibited prior to inhaling methacholine, even non-asthmatic subjects demonstrate bronchoconstriction.8,17–21 In the non-asthmatic, the resumption of deep inspirations rapidly reverses bronchoconstriction; however, marked attenuation of deep-inspirations-modulated bronchodilation is a feature of the asthmatic lung.8,17–20 In this patient, repeated deep inspirations from methacholine inhalation, lung volume, diffusing capacity, and FEV1 measurements, and even spontaneous sighs and coughs may have kept the airway-smooth-muscle in a relatively fluid state, limiting the change in FEV1 following methacholine inhalation.
The bronchoprotective effect of deep inspirations against methacholine in asthmatic patients in a clinical setting is not entirely clear. Todd et al10 studied 16 asthmatics whose PC20 was < 8 mg/mL, to assess the effect of deep inspirations (required in the methacholine challenge test with the dosimeter technique) on airway hyper-responsiveness. When the subjects inhaled methacholine to only half of their total lung capacity (ie, avoiding deep inspirations), the PC20 was markedly reduced, compared to when they conducted the methacholine challenge test with full deep inspirations (2.8 mg/mL vs 5.2 mg/mL, respectively, P = .02.). There was a more pronounced difference in a subgroup of subjects with the mildest asthma, who had a positive methacholine challenge result with submaximal inhalation of methacholine but a false-negative result (PC20 > 16 mg/mL) when they performed a standard methacholine challenge test with deep inspirations. The same group of researchers continued this line of investigation in a study that compared the dosimeter and tidal breathing methods2 of methacholine challenge test in asthmatic subjects.11 Twenty-four asthmatics underwent 3 different methacholine challenge test protocols: 2-min tidal breathing; 2-min tidal breathing plus 5 deep inspirations; and the standard 5 deep inspirations dosimeter technique. The spasmogenic effect of methacholine was markedly reduced with deep inspirations. Moreover, 6 asthmatic subjects with mild airway hyper-responsiveness had a negative methacholine challenge result when tested with the deep-inspirations dosimeter technique. Remarkably, one patient's PC20 increased to 174 mg/mL.11,12 In addition, Simard et al21 found that delaying post-methacholine deep inspirations (to measure FEV1) from 30 seconds to 3 min increased the fall in FEV1.
However, the literature is not universally supportive of the idea that deep inspirations are bronchoprotective in asthmatics. Kapsali et al22 studied the bronchoprotective effect of deep inspirations in asthmatic and non-asthmatic subjects. In the 8 subjects with asthma, 5 deep inspirations prior to methacholine inhalation had no effect on airway hyper-responsiveness, compared to without deep inspirations prior to methacholine inhalation. However, the response of only 8 subjects certainly cannot be extrapolated to every asthmatic patient one might encounter in a clinical setting. In addition, even though those subjects were classified as having mild asthma,1 the PC20 of every subject was < 1 mg/mL, indicating that they had more than mild airway hyper-responsiveness. The studies by Todd et al10 and Allen et al11 indicate that only patients with mild airway hyper-responsiveness are at risk of a false-negative methacholine challenge result because of the apparent bronchoprotective effect of deep inspirations. In addition, the subjects underwent a 20-min period of deep-inspirations prohibition prior to taking deep inspirations, so one could argue that the deep-inspirations prohibition period put the airway-smooth-muscle into a frozen state, making the cells less responsive to deep inspirations.
The potential pharmacologic influence of formoterol and budesonide on airway hyper-responsiveness must also be considered. My patient was taking formoterol/budesonide until 48 hours prior to testing. Withholding formoterol for 48 hours before testing conforms with the American Thoracic Society recommendations,2 but the clinician is dependent on the accuracy of the information provided by the patient. The American Thoracic Society guidelines for methacholine challenge test do not recommend withholding corticosteroids prior to methacholine challenge test, but do acknowledge that corticosteroids may reduce airway hyper-responsiveness.2 Indeed, budesonide creates a plateau in the maximum bronchoconstriction response to methacholine in asthmatics who previously demonstrated no limit to airway narrowing.23
In a study of fluticasone, airway hyper-responsiveness to histamine was significantly reduced and remained greater than baseline after a 2-week washout period.24 Mehta et al25 found reduced airway responsiveness to methacholine following treatment with mometasone, which was sustained throughout a 4-week washout period. In addition, inhaled corticosteroids restore the bronchoprotective effects from deep inspirations in patients with mild airway hyper-responsiveness.26
Taken in their totality, the data from my patient seem to support that he has asthma, despite the apparent negative methacholine challenge test. First, he has several asthma risk factors including atopy, genetic predisposition, variable FEV1, and dyspnea on exertion, which seemed to be intensified by a dusty work environment. In addition, the PFT data document FEV1 variability (see Table 3). His baseline FEV1 on the day of the methacholine challenge test had spontaneously declined 12.5% since the initial PFTs 17 months prior. Methacholine produced an additional decline from his initial value. That methacholine did not cause a more substantial FEV1 decline may be explained by the bronchoprotective effects of deep inspirations and budesonide. After bronchodilator, both the spontaneous and bronchoprovocation-induced FEV1 declines were completely reversed. Note that many clinics use only 2 puffs of albuterol to reverse the effect of the methacholine, and they allow 10 min prior to repeating the spirometry. The nebulized β agonist and anticholinergic certainly may have had an effect on the magnitude of the bronchodilator response. The post-bronchodilator FEV1 improvement was 16% above the pre-methacholine value, and 30% above the post-methacholine value. Had this patient's FEV1 fallen 22% after methacholine, the methacholine challenge test would probably have been interpreted in our lab as positive for mild airway hyper-responsiveness. The fact that this occurred from the combination of spontaneous bronchoconstriction and bronchoprovocation delivers the same diagnostic signal: a labile FEV1. The decision to formally diagnose asthma in a patient such as this would require additional information, such as the response to treatment.
Teaching Points
A restrictive pattern on spirometry does not preclude bronchodilator responsiveness. A restrictive pattern can be caused by asthma or other forms of reversible restriction.
In a patient with a history suggestive of asthma, normal spirometry cannot by itself rule out mild asthma, underlying airway hyper-responsiveness, or bronchodilator responsiveness.
Pulmonary function technologists need to be vigilant against technical and procedural factors that can produce a false-negative methacholine challenge result.
The act of breathing modulates airway-smooth-muscle tone. Bronchoprotection and bronchodilation from tidal breathing and deep inspirations are important to airway-smooth-muscle homeostasis. The benefits of deep inspirations are impaired in the asthmatic lung, but corticosteroids may return some responsiveness to deep inspirations.
In patients with mild asthma, the dosimeter technique for methacholine challenge test may produce false-negative results.
The effects of corticosteroids on airway hyper-responsiveness may persist for long periods following withdrawal of corticosteroids.
Footnotes
- Correspondence: Jeffrey M Haynes RRT RPFT, Department of Respiratory Therapy, St Joseph Hospital, 172 Kinsley Street, Nashua NH 03060. E-mail: jhaynes{at}sjhnh.org.
-
The author has disclosed no conflicts of interest.
- Copyright © 2011 by Daedalus Enterprises Inc.