Abstract
As the basis for this paper, it must be acknowledged that children are not simply small adults. But this acknowledgment must go further: infants are not simply small adolescents. As data for pediatric mechanical ventilation, in general, and the management for pediatric acute lung injury, more specifically, are very limited, the pediatric critical care clinician must closely assess the available adult data and evaluate its application for infants and children. Given the hurdles in studying pediatric acute lung injury and acute respiratory distress syndrome, clinicians involved with the care of critically ill infants and children are left with extrapolation of data from the neonatal and adult populations, reliance on the limited available pediatric data, careful assessment of the applicable physiologic and pathophysiologic principles, and/or reliance on their own experience and their colleagues' experience. Hopefully, with the collaboration of multicenter investigator networks, additional and definitive pediatric data may be on the horizon. In the meantime, sharing data between adult and pediatric populations seems to be an essential approach to the management of critically ill patients.
- mechanical ventilation
- pediatric
- acute lung injury
- gas exchange
- acute respiratory distress syndrome
- lung protection
- oxygenation
- ventilation
Introduction
The most common reason for admission to a pediatric intensive care unit (PICU) is the need for mechanical ventilation. Mechanical ventilation occurs on a daily basis for thousands of infants and children around the world.1–4 The reasons for mechanical ventilation are varied and include acute respiratory failure due to acute lung injury (ALI)/acute respiratory distress syndrome (ARDS), chronic respiratory failure due to neuromuscular weakness, acute neurologic abnormalities, airway protection for nonsurgical and surgical procedures, hemodynamic instability with the need to decrease oxygen consumption, and many others. However, definitive, randomized controlled trials to guide the pediatric critical care clinician in his/her ventilatory strategies for an individual infant or child are greatly lacking. Thus, one of the key questions that must be answered is whether data from the adult world can (and should) be extrapolated to infants and children. Conversely, is it reasonable to use pediatric data to guide practice for adult patients? The answer to both sides of the question is probably yes, with some constraints. Given the tremendous breadth of this topic, this paper will focus primarily on therapies/strategies available for the management of pediatric patients with ALI/ARDS. The definition of ALI/ARDS is based on the 1994 North American-European Consensus Conference and is generally accepted to be the same for infants, children, and adults.5–7
As the basis for this discussion, it must be acknowledged that children are not simply small adults. But this acknowledgment must go further: infants are not simply small adolescents. When deciding whether adult data can be safely and accurately extrapolated to pediatrics, one must ask critical questions about the population studied, the effect size, the disease process(es), and the pediatric patient or, more generally, the population in question. A large child or adolescent would clearly mimic the physiology of an adult more closely than would an infant. A primary difficulty for the pediatric practitioner becomes the question of an absolute cutoff age. Unfortunately, there is not a simple answer to this question, and the answer will probably change based on the clinical issue at hand.
Why Are Pediatric Mechanical Ventilation Data Lacking?
The Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network1–4 has helped to shed light on the reasons for the lack of definitive pediatric mechanical ventilation data, especially with regard to infants and children with ALI and ARDS. Nine North American PICUs were surveyed for all admissions over a 6-month period.1 Of 6,403 total admissions, only 1,096 (17.1%) required mechanical ventilation for greater than 24 hours. Furthermore, 701 of these patients met one of the generally accepted exclusions for a prospective clinical trial. These exclusions included limitations of life support, chronic ventilator dependence, chronic neuromuscular weakness, known upper-airway obstruction, hematopoietic stem cell transplantation, uncorrected congenital heart disease, and others. Of the 6,403 total PICU admissions screened over the 6-month period, only 395 (6.2%) were eligible for a mechanical ventilation weaning study,2 and 303 (4.7%) were actually enrolled. It is noteworthy that failure to obtain consent occurred in 23% of the eligible patients. Furthermore, of the 303 enrolled patients, only 23 were determined to have ARDS, representing 7.6% of the eligible patients and 0.4% of the total population screened. The most common diagnoses in this cohort were bronchiolitis (n = 81) and pneumonia (n = 48).
Whether the standard exclusion criteria used in the PALISI feasibility study1–2 are valid has become controversial. Should a prospective clinical trial study the real-life heterogeneous population or a more pure selected group of homogeneous patients? On the one hand, broadening the eligible population will probably increase enrollment, given the relatively small number of potential research subjects. However in doing so, the risk becomes the loss of a real signal in the noise of a more diverse population, and the risk of significant baseline differences in the groups studied. This later problem became apparent in a pediatric surfactant study3 in which a larger number of immunocompromised patients were randomly assigned to the placebo group, which eventually had a higher mortality. In such a situation, we are left with uncertainty whether the mortality difference was due to a real intervention effect or to baseline differences in the study groups. Although block randomization may be helpful, it is impossible to predict all potential group differences, and from a statistical viewpoint, the number of block randomized groups must be small.
In a more recent publication, Santschi et al, in collaboration with the PALISI Network and the European Society of Pediatric and Neonatal Intensive Care,4 found inconsistent mechanical ventilation strategies for pediatric ALI. Similar to the findings previously described,1 this larger-scale follow-up study included 59 PICUs in 12 countries in North America and Europe. Of 3,823 pediatric patients screened on 6 days separated by at least 4 weeks in 2007, 414 (10.8%) were diagnosed with ALI by the clinical care team at the individual centers. However, only 165 (4.3%) of the total patients screened met the pre-established inclusion/exclusion criteria for a clinical trial. Of these 165 pediatric patients, 124 (75.2%) were conventionally ventilated, 27 (16.4%) received high-frequency oscillatory ventilation (HFOV), and 14 (8.5%) were managed with noninvasive ventilation (NIV). Given these small numbers, the researchers concluded that randomized controlled trials in pediatrics would require international participation, with at least 60 centers. The required funding and cooperation would seem overwhelming to most investigators.
Khemani et al8 further defined the difficulties with pediatric mechanical ventilation studies by describing the characteristics of children who were intubated and mechanically ventilated across 16 North American PICUs. There has been substantial variability in the proportion of pediatric patients who are ventilated in various PICUs. This finding may have important implications when choosing PICUs for a clinical investigation. Criteria for intubation, patient populations, use of NIV, and severity of illness would probably become significant confounding variables. This variability may be, in part, due to the presence of step-down/progressive care units in the various pediatric facilities.
As a specific example of the inter-ICU variability seen by Khemani and colleagues,8 a pediatric study of PEEP may be very difficult to successfully accomplish. The vast majority of the infants and children were ventilated with a PEEP of 5 cm H2O. It is interesting to note that very few infants and children were ventilated with PEEP settings outside of the 4–8 cm H2O range. Reasons for the limited use of higher PEEP settings may include PEEP phobia, the low incidence of severe ALI/ARDS in the pediatric population, and/or the reasonably frequent conversion (as compared to the adult population) to HFOV as an alternative to a higher PEEP strategy. Furthermore, Figure 1 demonstrates the variability of PEEP settings within individual PICUs. Some centers were willing to vary PEEP on a case by case basis, whereas others rarely altered PEEP.
Variability of PEEP settings in individual pediatric intensive care units. Some centers were willing to vary PEEP on a case by case basis, whereas others rarely altered PEEP. (Adapted from Reference 8, with permission.)
Given these hurdles in studying pediatric ALI and ARDS, clinicians involved with the care of critically ill infants and children are left with extrapolation of data from the neonatal and adult populations, reliance on the limited available pediatric data, careful assessment of the applicable physiologic and pathophysiologic principles, and/or reliance on their and their colleagues' experience. As described in more detail below, hopefully, with the collaboration of multicenter investigator networks, additional and definitive pediatric data may be on the horizon.
Pediatric ALI: Learning From Adult Patients
Low-Tidal-Volume Ventilation
Current data indicate that limiting lung stretch should be the standard approach for all invasively ventilated adult patients with ALI/ARDS. However, there are insufficient data to prove that a tidal volume (VT) of 6 mL/kg (predicted body weight) is the ideal VT for all ventilated adult and pediatric patients. Until additional data become available, we must rely on the currently available evidence to guide our clinical practice, so low-VT ventilation with 6 mL/kg has become a generally accepted management strategy for ALI in adult patients. Many would argue that the only ventilatory intervention to conclusively reduce mortality (31.0% vs 39.8%, P = .007) for adult ALI/ARDS is low-VT (ie, 6 mL/kg predicted body weight) ventilation.9 In that landmark study, low-VT ventilation also increased ventilator-free days and number of days without failure of nonpulmonary organs or systems.
But definitive low-VT-ventilation data are lacking for infants and children. So for pediatric patients we must extrapolate the data from adult patients and/or rely on our clinical experience and very limited pediatric data. Some may argue that infants and children are different than adults and, thus, these low-VT data simply do not apply. However, the counter-argument for pediatric patients with ALI/ARDS is that the benefits of low-VT ventilation are real, whereas the risks are theoretical. Thus, a majority of pediatric intensivists have adopted low-VT ventilation for infants and children with ALI/ARDS.
So, the real question is whether low-VT ventilation should be employed for all pediatric (and adult) patients. Should low-VT ventilation be used for infants and children with congenital heart disease? What about for children with traumatic brain injury? More generally, what should be the ventilatory approach for any infant or child ventilated for a non-pulmonary reason with baseline normal lungs? We simply do not know the answers to these important questions.
Insights into the pathophysiology of ventilation-induced lung injury resulted from early pre-clinical studies that demonstrated that high-VT ventilation resulted in rapid pulmonary changes that mimic ARDS, despite baseline normal lungs.10,11 Ventilation settings that resulted in excessive pulmonary stretch led to the development of diffuse alveolar damage, with resultant pulmonary edema, increased inflammatory response, and leakage of these immune-modulators into the systemic circulation, with resultant multi-organ dysfunction/failure.12–17
Although multiple clinical investigations in adults ventilated with normal lungs18–27 have evaluated the effects of low-VT versus high-VT ventilation, most of those studies had significant intergroup differences in PEEP management and/or primary immune-modulator outcome measurements, of which the clinical correlation is uncertain. A study worth highlighting was by Gajic and colleagues,18 who suggested that most (and possibly all) mechanically ventilated adults should be managed with a “low”-VT strategy to avoid excessive lung stretch. The percentage of adult patients with previously normal lungs who developed ALI while mechanically ventilated is directly proportional to the size of the delivered VT. Patients with previously normal lungs who were ventilated with a VT < 9 mL/kg were much less likely to develop ALI while on mechanical ventilation than those adults who were ventilated with larger VT. However, it is important to note that the results of this study do not allow us to ascertain whether VT less than 9 mL/kg is less injurious than 9 mL/kg. It is unknown whether the less injurious effects of lower-VT ventilation are linear below 9 mL/kg or if a plateau effect occurs at lower VT. Unfortunately, additional data are not available to answer this clinically essential question.
Until a definitive randomized controlled trial is available in pediatrics, it would seem reasonable to ventilate infants and children with ALI or ARDS with a VT of 6 mL/kg predicted body weight. This recommendation is supported by retrospective pediatric data from Albuali and colleagues,28 which indicate that mortality among children with ALI was reduced by 40% with lower-VT ventilation. Two cohorts of patients were studied: those ventilated between 1988 and 1992, and those ventilated between 2000 and 2004. The patients in the earlier period were ventilated with higher VT (10.2 ± 1.7 mL/kg actual body weight vs 8.1 ± 1.4 mL/kg actual body weight, P < .001), lower PEEP (6.1 ± 2.7 cm H2O vs 7.1 ± 2.4 cm H2O, P = .007), and higher peak inspiratory pressure (31.5 ± 7.3 cm H2O vs 27.8 ± 4.2 cm H2O, P < .001). The more recent period had lower mortality (21% vs 35%, P = .04) and more ventilator-free days (16.0 ± 9.0 d vs 12.6 ± 9.9 d, P = .03). Lower VT was independently associated with lower mortality and more ventilator-free days, but the study had important limitations, including retrospective design, the measurement of VT at the expiratory valve of the ventilator (without consideration of the volume lost due to the distensibility of the ventilator circuit), and the calculation of VT based on actual (rather than predicted) body weight.
For pediatric patients with normal lungs, extrapolation from the available adult data would suggest ventilating with a VT less than 10 mL/kg. It should be noted that this recommendation is made upon extrapolation from the adult mechanical ventilation data, as reported by Gajic and colleagues.18
The bottom line is that low-VT ventilation definitely reduces mortality in adult patients with ALI. Adverse effects have not been reported in that population, and sedation requirements do not significantly increase. Thus, we are left with multiple questions for pediatrics. Can we obtain pediatric low-VT data? If so, would the results be different? Would it be ethical to conduct such a study? The ethical question is clearly linked to the proposed study design and populations. Many would have concern about studying 6 mL/kg versus 12 mL/kg, but what about 4 mL/kg versus 8 mL/kg, or 3 mL/kg versus 6 mL/kg? Additionally, what should be the age cohorts studied? As an underlying question, is there even equipoise for a low-VT ventilation study in pediatrics? Furthermore, what about specialized populations, including congenital heart disease and premature infants? What about infants and children ventilated for conditions other than ALI? Unfortunately, the clinical questions continue to greatly outnumber the available answers. With no definitive studies on the horizon, we remain reliant on extrapolation from the available adult data, in combination with our composite clinical experience and very limited pediatric data.
Tidal Volume Measurements
One important difference between ventilated pediatric and adult patients must be stressed as a component of any discussion involving VT delivery. For adult patients and larger pediatric patients, it is reasonable to measure the delivered VT at the expiratory valve of the ventilator, because the volume of gas lost due to the distensibility of the circuit is minimal, as expressed as a percent of the total VT delivered. However, for pediatric patients, especially for smaller children and infants, a substantial percentage of the delivered VT may be lost due to the distensibility of the ventilator circuit. Cannon et al29 found that for infants ventilated with a neonatal circuit, the expiratory VT measured with a pneumotachometer at the endotracheal tube is on average only 56% of that measured at the ventilator. Somewhat better correlation was seen in patients ventilated with a pediatric circuit, with the average measured VT at the endotracheal tube being 73% of that measured at the expiratory valve of the ventilator. Similar findings have been reported by Castle et al30 and Chow et al.31
Thus, when determining the actual delivered VT for smaller pediatric patients, placing a pneumotachometer at the endotracheal tube would seem to be the optimal approach. It must be noted that most of the newer generation of mechanical ventilators include software algorithms that estimate the actual delivered VT based on calculations using the compliance of the ventilator circuit; however, a review of the medical literature does not provide support that those algorithms have been systematically studied for the various devices and algorithms available. Heulitt et al32 studied one ventilator type and noted similar findings as previously reported, with the discrepancy between VT determinations again being greater for infants and smaller children than for larger patients. This study also noted that the differences in VT determination were affected by the use of a ventilator's circuit compensation algorithm. A key potential confounding variable is the potential change in the compliance of the ventilator circuit over time, due to temperature changes, secretions, condensation, and addition of external connectors.
A related topic is the increasing incidence of pediatric obesity. Thus, when determining the appropriate VT for a pediatric patient it is important to use predicted body weight. Although most standard textbooks include ideal body weight calculations for older children, adolescents, and adults, several Internet programs provide interactive calculators to determine the ideal body weight for pediatric patients as young as one year of age. Required inputs are sex, age, and height/length. Alternatively, one may estimate the ideal body weight for a child by using the available sex and age growth charts (http://www.cdc.gov/growthcharts/clinical_charts.htm). On the appropriate chart, graph the patient's height/length. Once the height/ length percentile is known based on sex and age, simply determine the predicted weight which corresponds to this same percentile.
PEEP
One of the most frequently discussed topics in the field of mechanical ventilation is PEEP titration. What defines optimal PEEP? What is the best practice for determining the optimal PEEP for an individual patient at a particular point in time? Several large-scale multicenter studies involving adults with ALI have attempted to address these issues. As with low-VT ventilation, the data on pediatric patients are very limited.
The ARDS Network's ALVEOLI PEEP trial33 by Brower et al, found that mortality was unchanged with the use of a more aggressive PEEP strategy, despite improved arterial oxygenation and improved pulmonary compliance. Several important points about this study should be stressed. First, the lower-PEEP group did not receive “low” PEEP, but, rather, “adequate” PEEP. Second, all of the enrolled patients, regardless of PEEP, were ventilated with a VT of 6 mL/kg and an end-inspiratory plateau pressure limit of 30 cm H2O. Additionally, there were no safety concerns noted in either of the study groups.
A follow-up study by Meade et al34 also found no survival benefit in adult patients with ALI/ARDS managed with a high-PEEP strategy and recruitment maneuvers, although, again, oxygenation was better. Mercat et al35 had a similar survival finding, but they found improved lung function, shorter duration of ventilation, and lower incidence of multi-organ failure with a more aggressive PEEP approach. It should be noted that all patients in the Meade and Mercat studies were also ventilated with a low-VT strategy.
In 2010, Briel et al36 extracted and combined the data from the Brower,33 Meade,34 and Mercat35 trials, which included 2,299 adult patients with ALI/ARDS. A higher-PEEP approach benefited adult patients with ARDS, with shorter duration of ventilation and lower hospital mortality.36 This benefit was not seen in patients who did not meet the standard accepted criteria for ARDS,5 and the study suggests that elevated PEEP may be associated with longer duration of ventilation in patients with less severe lung injury.
Although the data for adult patients are quite helpful, the pediatric critical care clinician is again left wondering about the best approach for infants and children with ALI/ARDS. Are the adult PEEP data applicable to infants and children due to their differences in chest wall compliance, cardiac reserve, sedation requirements to tolerate mechanical ventilation, and others? Unfortunately, there is no clear answer to this question. It would seem likely that the adult PEEP data would be applicable to an otherwise normal older child or adolescent, but probably not to an infant with congenital heart disease. Thus, the clinician caring for the pediatric patient must carefully consider the physiology and pathophysiology involved before determining the applicability of the available data from adult ALI and ARDS patients.
In looking to the future, will we be able to obtain definitive PEEP data for infants and children? What PEEP-FIO2 table should be used in the design of a pediatric PEEP trial? Many pediatric clinicians would argue that the PEEP-FIO2 table used in the adult studies33–35 would be too aggressive for infants and children. Is this belief simply PEEP phobia or is it realistic given the cardiorespiratory status of infants and children in relation to that of adults? The answer probably lies somewhere in between these possibilities. Given the relatively low mortality in pediatrics, as compared to adults, is mortality a realistic end point, given the limited number of pediatric ARDS patients? It seems unlikely to power a pediatric ARDS-PEEP study on mortality as a primary end point. The most realistic end points for such a study would probably need to be ventilator-free days and organ-failure-free days.
As the most valid PEEP studies in adult patients standardized VT in the control and intervention groups at 6 mL/kg predicted body weight, would a pediatric low-VT study need to occur before a pediatric PEEP study? The questions again become more numerous than the available answers. For now, pediatric critical care clinicians are again left with extrapolating data from the adult population, relying on individual and institutional experience, and a careful assessment of the specific physiology and pathophysiology involved.
Fluid Management in Acute Lung Injury
Diuretics are frequently administered in the critical care setting to adult and pediatric patients, for many conditions. The data to support diuretic use in adult patients with ALI/ARDS originate from another ARDS Network randomized controlled trial.37 Although no survival benefit was demonstrated between a conservative and a liberal fluid-management strategy, the conservative fluid-management approach increased the number of ventilator-free and ICU-free days. Of importance, an increase in renal failure was not seen in the conservative fluid-management group. Thus, the use of diuretics has become a relatively common approach in this patient population.
Although definitive pediatric fluid management does not exist, a general approach to the infant or child with ALI is similarly to use diuretics with a conservative fluid-management strategy in mind. The PALISI Network1,2,38 is currently considering such a study. Key issues for a clinical fluid-management investigation include the fluid target(s) (eg, in/out fluid balance, central venous pressure, daily weights). In preliminary work the PALISI network found that cumulative fluid balance for pediatric ICU patients with ALI does not have clinical utility.38 Thus, the key to success of such a study will be agreement among the investigators on the liberal and conservative fluid-management algorithms, use of invasive monitoring lines, fluid titration end point(s), and outcome measure(s).
Until pediatric data become available, it seems reasonable to extrapolate the general ARDS Network approach to pediatric ALI/ARDS patients and utilize a conservative fluid approach. Unfortunately, the ARDS Network's algorithms37 have not been generally applicable to most pediatric populations.
Corticosteroids for Acute Lung Injury
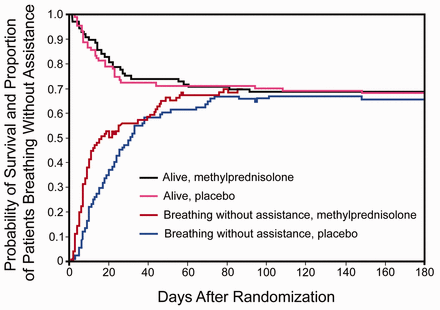
The use of corticosteroids for ALI/ARDS in both the adult and pediatric populations remains controversial.39 The ARDS Network's study40 yielded several conclusions. Methylprednisolone was associated with significantly higher 60-day and 180-day mortality in patients enrolled 14 or more days after developing ARDS, whereas mortality was no different in the patients enrolled less than 14 days after ARDS onset. Methylprednisolone increased the number of ventilator-free and shock-free days during the first 28 days (Fig. 2). As compared with placebo, methylprednisolone did not increase the overall rate of infectious complications but was associated with a higher rate of neuromuscular weakness.
Corticosteroid therapy for persistent ARDS does not affect survival but does increase ventilator-free days. (Adapted from Reference 40, with permission.)
In a meta-analysis by Tang et al,41 corticosteroids were associated with better survival and less morbidity, without adverse effects. Tang et al concluded that the consistency of the results, in terms of study designs and outcomes, suggests that corticosteroids are an effective treatment for ALI/ARDS. It should be noted that the studies of corticosteroids in the management of adult ALI have methodological limitations, including relatively small sample size. Recommendations differ among experts regarding corticosteroids for late-stage ARDS, although there appears to be a trend toward recommending low-to-moderate-dose corticosteroids for acute lung disease of less than 14 days. It seems reasonable that if corticosteroids are administered, infection surveillance, gradual taper of corticosteroids, and avoidance, if possible, of neuromuscular blockers should occur.39
Given the somewhat conflicting results of the ARDS Network40 and Tang et al41 studies, the extrapolation of these findings to pediatrics is less clear than for low-VT ventilation or PEEP titration. Although the use of corticosteroids in the PICU setting seems to be increasing for a variety of critical care disorders, most commonly shock, it must be noted that the use of corticosteroids for pediatric ALI remains at the discretion of the clinician, because definitive data are clearly lacking. Given the more pressing clinical questions of lung-protective ventilation, PEEP titration, and fluid management, it is uncertain if/when the role of corticosteroids for pediatric ALI will reach the top of the list for a multicenter prospective randomized clinical trial.
Pediatric and Adult Acute Lung Injury: Synergistic Data
Despite the differences between the pediatric and adult populations, there are several adjunct therapies for ALI that have yielded similar data. The most commonly discussed (and most controversial) of these approaches are exogenous surfactant administration and inhaled nitric oxide (INO).
Exogenous Surfactant Administration
Despite the fact that exogenous surfactant administration is a standard of care for infants with neonatal respiratory distress syndrome,42–46 its use for pediatric and adult ALI remains debated. Although the preliminary results47–49 of surfactant administration for pediatric ALI were promising, more recent studies have been disappointing.3,50,51 Similarly disappointing have been the results of surfactant administration in adults.51
For adults with ALI/ARDS, exogenous surfactant administration did not improve outcome and showed a trend toward higher mortality and more adverse effects. Kesecioglu and colleagues concluded that at this time exogenous surfactant cannot be recommended for routine use in adult patients with ALI/ARDS, but that alternative exogenous surfactant preparations and/or dosing regimens may prove to be beneficial.51 A discussion of the various exogenous surfactants at various stages of development and investigation is beyond the scope of this paper.47,52–58
Based on both pediatric and adult studies, the use of surfactant for non-neonatal ALI remains uncertain. Although many had hoped that the Calfactant Therapy for Direct Acute Respiratory Distress Syndrome and Direct Acute Lung Injury in Children (CARDS) trial, which involved 30 international centers, would provide definitive guidance for the management of patients with direct lung injury, the study was recently closed for futility in both the pediatric and adult arms.52
A more comprehensive discussion of exogenous surfactant use is beyond the focus of this article. For a more detailed review of surfactant use in the PICU setting as well as for speculations about the future of this potential therapy, please see Willson and Notter's paper in this issue.52
Inhaled Nitric Oxide
INO plays an important role in the management of infants with persistent pulmonary hypertension of the newborn, older children and adults with pulmonary hypertension, and a subset of patients with congenital heart disease. However, despite several studies in both pediatric and adult patients that found clear improvements in oxygenation with INO therapy,59–62 improvements in measurable outcomes have not been demonstrated.61–64 Many have hypothesized that INO should benefit patients with ALI by improving oxygenation and thus allowing the clinician to wean ventilatory support and minimize the occurrence of ventilator-induced lung injury. Unfortunately, this theory has never been proven correct.
In a meta-analysis of 14 randomized controlled trials with a total of 1,303 participants, INO showed no statistically significant effect on mortality.63,64 Studies have failed to demonstrate a statistically significant effect of INO on mortality, duration of ventilation, ventilator-free days, ICU stay, or hospital stay. Of interest, INO may increase the risk of renal impairment in adults.63 Afshari et al63,64 concluded that INO cannot be recommended for patients with acute hypoxemic respiratory failure. INO transiently improves oxygenation but does not reduce mortality and may be harmful.63,64 Based on the currently available data, this conclusion seems applicable to both children and adults with ALI/ARDS, in the absence of clinically important pulmonary hypertension.
Adult ARDS: Learning From Pediatric Patients
Extracorporeal Membrane Oxygenation
Extracorporeal membrane oxygenation (ECMO) is one therapy on which there are substantially more data from infants and children than from adults. ECMO involves an extracorporeal circuit with a pump and an oxygenator, to support life in the case of refractory cardiac and/or respiratory failure. In venoarterial ECMO, both the heart and lungs are bypassed to provide cardiac and/or respiratory support. In venovenous ECMO, the ECMO system serves as an additional lung to augment gas exchange, thus allowing the ventilator settings to be set below (ideally, substantially below) toxic levels. With venoarterial or venovenous ECMO the clinician must remember that ECMO does not heal or treat the underlying problems but rather provides cardiac and/or respiratory support to allow the underlying processes to resolve without the need for toxic ventilator or vasoactive agent/inotrope support. Please see Dalton's paper in this issue for a more comprehensive discussion of ECMO.65
Although the initial (and successful) use of ECMO occurred in an adult trauma patient, this life-saving technology became a standard approach for refractory cardiorespiratory failure in the neonatal and, subsequently, pediatric populations long before being established as a potential therapy for adults.66–73 Of the 44,824 patients who have been reported to the Extracorporeal Life Support Organization from the late 1980s through December 2010, 65.2% have been neonates, 25.0% have been pediatric patients, and 9.8% have been adults.74 For those patients requiring respiratory support, survival is clearly better for neonates (75%) than for pediatric patients (56%) or adults (54%) (Table 1). It is interesting to note the very similar ECMO survival rates for pediatric and adult patients requiring respiratory support.
Numbers and Outcomes of Neonatal, Pediatric, and Adult ECMO Patients, as of December 2010
Although ECMO support for refractory hypoxemia has become routine for neonates, infants, and children, the extrapolation of this experience to adults has increased only in recent years. The H1N1 experience72,75 and the positive results of the European Conventional Ventilation or ECMO For Severe Adult Respiratory Failure (CESAR) trial for refractory ARDS emphasize the potential benefits of ECMO in adults. ECMO highlights an important area in which neonatal and pediatric critical care clinicians can share not only data but also important experience with our adult colleagues.
High-Frequency Oscillatory Ventilation
HFOV is another management strategy for ALI/ARDS that has expanded from pediatrics to adults. Despite the lack of a definitive, randomized controlled trial in pediatric patients to demonstrate an advantage of HFOV over a conventional-ventilation, low-VT strategy, the use of HFOV in infants and children has become routine.76–78 The one randomized controlled trial of HFOV and conventional ventilation was reported back in 1994 by Arnold et al, and it should be stressed that the control group received a high-VT approach.79
In the adult population, HFOV has been demonstrated to be equivalent to lung-protective, conventional ventilation, and its use has steadily increased.80–82 However, despite the available data and increasing use, HFOV for adult ALI/ARDS remains controversial, and this controversy is well described in Fessler and Hess's paper from a prior Journal Conference.83
The question of whether definitive data showing the superiority of HFOV over conventional ventilation will be obtained for children or adults remains uncertain. With the widespread use of HFOV in pediatrics, it seems unlikely that such a study will be performed, as equipoise may be lacking, especially in centers that routinely use HFOV. Without those, generally larger, centers there may be an inadequate available population for such a study. It also remains uncertain whether the ARDS Network will embark on such a study in the adult population. In the meantime, it is worthwhile to mention a meta-analysis published in the British Medical Journal last year84 that concluded that HFOV “might improve survival and is unlikely to cause harm.” Unfortunately, that may be the best summary we will have on HFOV for pediatric or adult ALI/ARDS for some time to come.
Summary and Thoughts for the Future
Prospective randomized controlled trials on pediatric ALI and ARDS are needed. In the absence of definitive data, pediatric critical care clinicians are left with extrapolation of data from the neonatal and adult populations, reliance on their and their colleagues' clinical experience, and the clinical application of the pertinent physiology and pathophysiology. As the available data, clinical experience, and knowledge of the physiology/pathophysiology are extrapolated to a specific patient, one must remember carefully to consider the numerous uncontrolled variables in the “real life” critical care setting, including the knowledge and experience of the respiratory therapists, physicians, nurse practitioners, bedside nurses, ECMO specialists, pharmacists, and the other personnel in that ICU team.
Until definitive data are obtained to answer the many clinical questions posed in this article in regard to the pediatric population, we must remember that often more data are required to determine the efficacy of a therapy than its safety. Thus, one must consider the frequently debated question, if an approach has been demonstrated to be safe but is unknown to conclusively improve outcome, is it reasonable to proceed? In a paper from a prior Journal Conference, Rubin and Steinberg85 concluded that:
Physicians are morally obligated to provide the best and most appropriate care possible for their patients, but when accepted approaches are failing and a critically ill patient is getting worse, the critical care physician must make a decision regarding innovative therapy, based on the patient's prognosis, the available evidence, the resources on hand, the expertise of the physicians, and the values of the patient and the physician. This decision may lead, at times, to trying unproven and innovative strategies to achieve a clinical goal. In such cases, it is to be hoped that this can be done in such a way that data are formally and prospectively collected to increase our knowledge.
One should remain hopeful that more definitive pediatric data are forthcoming, especially with the cooperative efforts and recent growth of the PALISI Network,1–3,38 the Collaborative Pediatric Critical Care Research Network,86–89 the Australian and New Zealand Intensive Care Society Clinical Trials Group,6,90,91 and other multicenter collaborations. We look forward to more definitive data to guide pediatric critical care clinicians in their management of infants and children with ALI and ARDS are on the horizon.
Discussion
Fineman:
In terms of outcomes in younger kids, obviously mortality is not very high, but are ventilator-days really that important? Should we be looking more at longer-term functional outcomes? Are those easy to do?
Cheifetz:
We cannot target mortality in an ALI/ARDS study, because the mortality is too low to significantly change with a single-intervention study. I believe ventilator-free days and ICU-free days are reasonable end points. If a patient is going to survive, we all know that every family, every patient, everyone wants this to occur as quickly as possible. Also, from the viewpoint of resource utilization, ICU days and ventilator days are important.
Your second question raises a very important point. We have not done a good job of determining neurologic and pulmonary function at 6 months, 12 months, and beyond. Most would agree that it is acceptable to be on a ventilator in the ICU for 5 additional days if that provides a better functional outcome at 6 or 12 months. But we have no data, and it is an important point that we need to consider as we design future studies.
Fineman:
Everyone talks about whether adults and kids should be put in the same trial, but what about an 8-month-old and a 14-year-old?
Cheifetz:
That is a key point. Though children are not simply small adults, there is an age at which the physiology becomes sufficiently similar that we can combine older children and adults. Most would agree that an 8-month-old and a 14-year-old are very different. The FDA has used an age cutoff of 12 years for including pediatric patients in adult studies. I think that's reasonable, but I do not know what the cutoff should really be. Is it really 12 years? Should it be 10 or 14 years? We will wrestle with this question as we design future studies.
Walsh:
Chest-wall compliance seems to be different between children and adults, but you didn't mention anything about measuring transpulmonary pressure.
Cheifetz:
That is important, but I promised I would stick to my 30-minute time limit. It comes back to the physiologic differences based on age. Chest-wall compliance is an important variable if you are studying compliance changes in a PEEP study.
Myers:
In Colorado about 7 years ago, some of us raised these same questions. I think it's just the lack of funding that's preventing the multicenter pediatric trials we need. It just can't be done in a single center. The pediatric data from Cannon et al1 was over a decade ago, and they used Servo 300 ventilators. Ventilators and ventilator circuitry have greatly changed over the past decade. Do you think that the scattered VT measurements would still be the same today if that study was repeated?
Cheifetz:
We debate that topic in our institution as well. Is the current generation of ventilators, with their improved algorithms, better than their predecessors? In preparing for this presentation, I hated to pull up a 10-year-old study, but I could not find any definitive data in the last 10 years. I think it's extremely important to study that question. In a low-VT study in infants and children it would be essential to compare VT measurements from the ventilator (with the various algorithms that compensate for ventilator circuit compliance) and VT measurements obtained at the endotracheal tube. Although ventilator algorithms are clearly better than 10 years ago, I remain skeptical that any algorithm could be as accurate as measuring the VT at the airway. There are too many variables that can change during the course of ventilation, including temperature, humidity, secretions, and airway/circuit adaptors.
Curley:
A lot of what you presented is operator-dependent. We know there is wide variation at the bedside. Half of what we think we're doing is even being done, let alone done well. I had one clinical site that had a very high enrollment rate during the mock screening and then went down a lot during the trial. A year later I called them up and asked what was going on. One thing that had changed was that they were using more NIV than ever before, which was preventing kids from being intubated. They were able to support pretty sick kids on NIV, which takes away the entire high-versus-low-VT question.
As far as outcomes are concerned, it depends on the question. We have ventilator-free days and organ-failure-free days, and we can all agree on an operational definition, but that demands the protocols and good clinicians implementing the protocols. But we also have to do long-term follow-up in our patient population. And when we do follow-up, a 14-year-old is different than a 2-year-old: we use different metrics. Being in an ICU is traumatic for kids and the entire family.
Cheifetz:
I completely agree. Our patient populations are so heterogeneous, and the population of any ICU can substantially change over time. You know this as well as anyone. Look how long it took to launch the RESTORE [Randomized Evaluation of Sedation Titration for Respiratory Failure] sedation trial [http://www.restorenetwork.org/default.aspx]. I believe you described an 11-year process. Populations, ICUs, management strategies, et cetera will change over that time frame, so we need to consider such moving targets. We always have to consider the uncontrolled variables.
Willson:
On the problem of practice variation, in my unit we have nurses, respiratory therapists, physicians, residents, and fellows all playing with the ventilator. They're not supposed to, but they do, so the practice variation is tremendous. We just completed another surfactant study, and there was a protocol, but I'm not sure it made any difference in terms of outcomes and how patients were managed.
Before we address any of these questions, my plea would be to get it down to an adequately explicit methodology. Let's put it on a computer. Let's allow people to make ventilator adjustments but require that they justify what they did. Phillipe Jouvet, in the Department of Pediatrics at University of Montreal, started with a simple computer protocol that sits next to the ventilator, and every time you make an adjustment you have to justify why you did it. Before we do anything else, that has to be done, because we don't even begin to understand the practice variation that's involved.
Rubin:
I'm going to take up your challenge. You threw down the gauntlet that if something has no proven benefit but it appears to be safe, why shouldn't we use it? I would argue vigorously that there's a very good reason not to do that. First, safety is difficult to test. You gave the example that heliox appears to be safe in all studies, but none of the studies were actually designed to evaluate safety. Just one serious adverse event related to an intervention that has no proven benefit is one too many. We may not identify those rare, adverse events until we look for them and have a large number of patients.
And when you talk about safety, it's not just patient safety, there's a lot of other things that go into this: confounding variables of additional therapies and attribution of benefit when benefit may not exist or may be due to parallel interventions. You can say that heliox is benign, but now I have to think about how we're giving our aerosol therapy because it's a different viscosity, and how we are adjusting the ventilators. And then there is the additional cost. I would argue strongly against using things without proven value, unless it's within the context of a trial.
Cheifetz:
I thought you or Rich [Branson] might take me up on that. I completely respect that view, but I will argue the other side. We simply do not have definitive data, especially in pediatrics, for many therapies in our armamentarium. Should we not offer a therapy that has real potential to help and minimal potential to hurt simply because definitive data are lacking?
Heliox is an example. There have been multiple studies, which I acknowledge were not designed with safety as a primary end point, but, still, none of the studies even hinted at any safety concerns. Thus, we have supportive, but not definitive, data, due to study-power limitations, supportive physics and physiology, and no evidence for safety concerns. So should we not use this therapy when the clinical experience is that at least some patients clearly benefit? Should a clinician withhold such a therapy when the best available information suggests that it is safe and may provide benefit, but it simply has not had the perfect study to prove it?
Consider the simple example of the parachute.1 There has never been a prospective randomized controlled trial that demonstrates that a parachute really works, but no one would ever question its effectiveness or safety profile, at least with the respect to what would be the obvious control group.
Rubin:
Yeah. Do the right study then. If you can identify that subpopulation and you know the benefit clinically, then God bless you and use it. But what is to prevent us from extending it to patients with a variety of diseases just because we don't have data? What's to prevent us from using a whole series of drugs just because? I would argue that the more beneficent way of doing this is that if you're faced with these sorts of decisions, don't just do something: stand there. Stand there and think.
Cheifetz:
I could not agree more that as clinicians, when data are lacking, we should stand still for an appropriate amount of time and think before simply acting. Beyond that, if we don't have definitive data, as is often the case, then we must rely on our clinical experience, clinical interpretation, and extrapolation of the available data. I believe we would both agree that this discussion can become very theoretical and could go back and forth many times.1 The obvious question is, why not study a subpopulation where a specific therapy seems to work? Well, it comes down to the usual suspects: time, sample size, and funding. As we do not have enough time to fully debate this issue, I would refer the audience to read the excellent paper in which you debated this topic with Ken Steinberg in the proceedings from a prior Journal Conference.1
Brown:
Regarding practice variation at the beside, designing studies, and evaluating techniques, I think something physicians frequently miss in this discussion is the complexity of modern ventilators and how many adjustments the respiratory therapist can make that are not physician-ordered variables, and how dramatically your ventilation and oxygenation can change after making those changes. It's quite profound. I think when we design a study to evaluate what ventilator settings they were on, we really have little idea what ventilator settings they were on.
Cheifetz:
You are absolutely correct. With a modern ventilator the respiratory therapist can essentially change what a mode is doing (or even functionally change between modes) by using sub-menus and advanced options and settings to sculpt the breaths. They can change almost everything about a breath without the changes being controlled by protocols or physician order.
Curley:
I think we can do this, because if we could agree about what we know right now, and everyone could agree in principle how on to ventilate a patient, as new therapies are developed we could systematically evaluate them. I think that the PALISI Network could, if given a minimum data set and point prevalence surveys, describe and monitor what people are actually doing. Given the electronic case report forms, it doesn't take a lot of information. We typically want every data element on every patient, which requires a lot of time and money, but in reality I think we could do more by forming networks, agreeing what the floor is, practicing the floor, and doing minimum data capture to test whether different things work in subpopulations where it makes sense.
Cheifetz:
I could not agree more. Absolutely.
Gentile:
Going back to adults versus pediatric patients, the ARDS Network is a clear demonstration of how progress is made. After the positive low-VT trial, everybody ran to the ventilator and turned the knob to the left, but VT is based on predicted body weight, and we are all getting bigger, including kids. You pointed out that volume controlled continuous mandatory ventilation can be very uncomfortable. And the ICU sedation, analgesia, and delirium clinical trials showed that we should let patients breathe spontaneously. Volume controlled continuous mandatory ventilation at 6 mL/kg predicted body weight is lung-protective, but what happens when the patient breathes on their own? If we simply put an endotracheal tube in and let someone breathe, the VT can't be controlled or limited, except with sedation.
Cheifetz:
Good point. In pediatrics we should not strive to reinvent the wheel. We should base future clinical mechanical ventilation trials on what is already known, often from neonatal and adult patients, learn from the experience of others, and then extrapolate. I think your comment is important.
Gentile:
Martha [Curley] touched on patients managed with NIV. Many ICU-based mechanical ventilation trials are now having problems meeting enrollment goals because those patients are now on NIV, and not in the ICU, which leaves the ultra-sick patients in the ICU, who tend to meet the exclusion criteria for various reasons.
Cheifetz:
NIV in pediatrics is a huge challenge. Not only do you have the same issues as in adults, but you have the additional issue of interfaces for our various sizes of patient, plus a lack of FDA-approved devices.
Curley:
Regarding clinician adherence to protocols, the reason we did the prone-positioning study is that we thought there would be differences in kids. But we ended up stopping the trial. I know we're not supposed to talk about post-hoc analysis, but there was an improved mortality in kids who were better, as compared to the findings of Gattinoni, who found better outcomes in worse patients. There might be a difference in pediatric patients based on physiology. I think we have to continue to ask questions specific to pediatrics, where they make sense.
Walsh:
Can we translate some adult medicine studies to pediatrics? One of the classic indicators of ARDS is a PaO2/FIO2 of < 200 mm Hg. Many of our kids probably have a lower PaO2/FIO2, and we just don't know about it because they typically don't have an arterial line, the typical indication for which is hemodynamic instability, not gas exchange. When using an SpO2/FIO2 of < 235 mm Hg to identify patients, I was surprised that there were a lot of patients who would have met the ARDS criteria if we had arterial blood gas values.
Cheifetz:
The use of arterial lines in the PICU settings seems to vary over time. There are data1,2 that have validated the use of SpO2/FIO2, but I'm not sure how much SpO2/FIO2 has caught on in standard clinical practice. Is it reasonable to design a low-VT study using the SpO2/FIO2 as an entry criterion, and would we have buy-in from clinicians? I don't know. Would you accept a clinical ARDS study using SpO2/FIO2 as either an inclusion criteria or a secondary outcome measurement?
Gentile:
As long as the patient is not intubated, yes. But the oxygenation index is a much clearer indication of lung impairment, because a pressure cost of oxygenation is reflected in the specific amount of ventilator support. This is one of the things that was developed in pediatrics but is used with increasing frequency in adults. I think it's very important to obtain the oxygenation index, because if the patient is on a PEEP of 5 cm H2O, compared to a PEEP of 20 cm H2O, there's a huge difference in severity of lung injury that's not reflected in PaO2/FIO2.
Willson:
I love the idea of using noninvasive measurements, and indeed we do. We've seen a tremendous drop in the number of arterial lines. The problem with the surfactant study was that people insisted that SpO2 had to be 100% or they're just not comfortable. Of course when you then use the SpO2/FIO2—and you can do the same with the oxygenation index, substituting saturation for PaO2—but it's only reliable if the SpO2 values are in the range of 90–96%. It's really discouraging when people have agreed to use the saturation rather than the PaO2 but tend to keep patients 100% saturated. It's a constant battle.
Unfortunately, in our study, where we're looking back at the data, I dare say that over half of the SpO2 values are 100%, and calculating a saturation index or a saturation ratio doesn't make any sense. Good conceptually, but until we get people to turn down the FIO2 so SpO2 is 90–96%, then using SpO2 rather than PaO2 is just not going to work.
Cheifetz:
Finally, I want to ask you this question. Can we study low VT in infants and children? Do you believe we have sufficient equipoise? Could we successfully complete that study? Most of us would probably agree that we should do such a study, but can we? Is it realistic? From the show of hands, it is a 50/50 split.
Rubin:
Well, it's not just yes or no. It's yes, no, or it depends on study design. If you're doing 6 cm H2O versus 12 cm H2O, the answer is no. If you're doing 4 cm H2O versus 8 cm H2O, the answer is perhaps.
Cheifetz:
Agreed. So please answer the question assuming the study would be designed exactly how you personally believe it should be. I realize that there are many variables, but if you have the right study group, should this study be attempted, and can it be successfully completed? With this clarification, who believes that such a study is doable? Still about half: maybe slightly more than half.
What about PEEP? Should we? Could we? Same assumptions: with the right algorithms and a protocol that you would accept, can/should we do a PEEP trial in pediatrics? A little less, about one third are raising their hands.
Footnotes
- Correspondence: Ira M Cheifetz MD FAARC, Pediatric Critical Care Medicine, Duke Children's Hospital, Duke University Medical Center, Box 3046, Durham NC 27710. E-mail: ira.cheifetz{at}duke.edu.
Dr Cheifetz presented a version of this paper at the 47th Respiratory Care Journal Conference, “Neonatal and Pediatric Respiratory Care: What Does the Future Hold?” held November 5–7, 2010, in Scottsdale, Arizona.
Dr Cheifetz has disclosed relationships with Philips-Respironics, Covidien, Discovery Laboratories, and Teleflex.
- Copyright © 2011 by Daedalus Enterprises Inc.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.
- 14.
- 15.
- 16.
- 17.↵
- 18.↵
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.
- 44.
- 45.
- 46.↵
- 47.↵
- 48.
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.
- 54.
- 55.
- 56.
- 57.
- 58.↵
- 59.↵
- 60.
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.
- 68.
- 69.
- 70.
- 71.
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.
- 78.↵
- 79.↵
- 80.↵
- 81.
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.
- 88.
- 89.↵
- 90.↵
- 91.↵