Abstract
Since the identification of surfactant deficiency as the putative cause of the infant respiratory distress syndrome (RDS) by Avery and Mead in 1959, our understanding of the role of pulmonary surfactant in respiratory physiology and the pathophysiology of acute lung injury (ALI) has advanced substantially. Surfactant replacement has become routine for the prevention and treatment of infant RDS and other causes of neonatal lung injury. The role of surfactant in lung injury beyond the neonatal period, however, has proven more complex. Relative surfactant deficiency, dysfunction, and inhibition all contribute to the disturbed physiology seen in ALI and acute respiratory distress syndrome (ARDS). Consequently, exogenous surfactant, while a plausible therapy, has proven to be less effective in ALI/ARDS than in RDS, where simple deficiency is causative. This failure may relate to a number of factors, among them inadequacy of pharmaceutical surfactants, insufficient dosing or drug delivery, poor drug distribution, or simply an inability of the drug to substantially impact the underlying pathophysiology of ALI/ARDS. Both animal and human studies suggest that direct types of ALI (eg, aspiration, pneumonia) may be more responsive to surfactant therapy than indirect lung injury (eg, sepsis, pancreatitis). Animal studies are needed, however, to further clarify aspects of drug composition, timing, delivery, and dosing before additional human trials are pursued, as the results of human trials to date have been inconsistent and largely disappointing. Further study and perhaps the development of more robust pharmaceutical surfactants offer promise that exogenous surfactant will find a place in our armamentarium of treatment of ALI/ARDS in the future.
- surfactant
- infant respiratory distress syndrome
- RDS
- acute lung injury
- ALI
- acute respiratory distress syndrome
- ARDS
- neonatal
Introduction
Exogenous lung surfactants are among the most studied drugs in medicine. Surfactant therapy is currently a mainstay in neonatal care, where its use has been associated with a significant reduction in the morbidity and mortality accompanying premature birth. Given the physiologic necessity of pulmonary surfactant for normal breathing, and its demonstrated deficiency in prematurity, the efficacy of exogenous surfactant therapy in pre-term infants is predictable. There is also evidence of surfactant dysfunction in many forms of acute pulmonary injury in term infants, children, and adults as well, but the evidence of therapeutic efficacy for exogenous surfactant outside of the neonatal period is more limited.
This review discusses the evidence for surfactant dysfunction in acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS), and clinical studies to date of exogenous surfactant therapy in term infants, children, and adults. While therapeutic use of surfactant in acute pulmonary injury outside of the neonatal period enjoys biologic plausibility and has achieved success in treating term infants and children with several direct types of acute pulmonary injury, controlled clinical studies have been largely unsuccessful in adults with ALI/ARDS. Possible reasons for this and future directions in continuing surfactant research will be discussed.
A Brief History
If you would understand anything, observe its beginning and its development.
— Aristotle
The phenomenon of surface tension and its effects on the pressure drop across an interface separating 2 phases of matter was defined mathematically in the early 1800s by the Law of Young and Laplace. Applied to the pulmonary alveoli, this law states that the pressure drop required to inflate or maintain these air sacs at a given size is directly proportional to their surface tension and inversely proportional to their radius. The work of breathing, which generates the necessary pressure drop, is thus similarly directly proportional to surface tension. The important contribution of surface tension forces to lung mechanics was documented in 1929, when von Neergaard1 showed that it took higher pressure to inflate excised animal lungs with air (when surface tension was present) than with saline (when surface tension forces were minimal because there was no longer a liquid-air interface). Analysis of these data indicated that over half of the pressure drop needed to inflate the normal lung was to overcome surface tension forces.
An equally important fact not appreciated at the time of von Neergaard's experiments was that pulmonary surface tension forces would actually be much larger (and in fact physiologically unsupportable) if surface tension in the normal lungs were not greatly reduced by lung surfactant. Surface-active substances (surfactants) lower surface tension biophysically by virtue of an amphipathic (polar/non-polar) molecular structure, and the presence of surfactants in the lungs is essential for normal respiration. The existence of lung surfactant in air-breathing animals was documented in the mid-1950s by Pattle2,3 and Clements.4 This complex material, a mixture of lipids and specific proteins, is one of the most powerful surface-active substances known. Table 1 shows representative compositional percentages for large aggregate surfactant isolated from alveolar washings via sedimentation and density-gradient centrifugation or type II cell lamellar bodies from multiple animal species.5 The major phosphatidylcholine fraction of total surfactant phospholipid is about 55% saturated (dipalmitoylphosphatidylcholine plus other saturated phosphatidylcholines) and 45% unsaturated. The anionic phospholipids group includes phosphatidylglycerol, phosphatidyl-inositol, and phosphatidylserine. Other phospholipids include phosphatidylethanolamine and sphingomyelin. Neutral lipids include cholesterol and cholesterol esters plus diglycerides/triglycerides.
Composition of Natural Surfactant (%)
By lowering and varying alveolar surface tension, lung surfactant maintains a low physiological work of breathing, stabilizes small alveoli against collapse (atelectasis), improves the overall uniformity of alveolar inflation, and reduces the hydrostatic driving force for pulmonary edema. Details of the discovery, composition, and physiological actions of pulmonary surfactant are reviewed elsewhere.5,6
Because of its necessity for normal respiration, a deficiency or dysfunction of lung surfactant results in severe respiratory failure. Soon after the discovery of lung surfactant, Avery and Mead7 in 1959 suggested that surfactant deficiency might account for infant respiratory distress syndrome (RDS). Subsequent studies identified the most prevalent phospholipid component of lung surfactant as dipalmatoylphosphatidylcholine (DPPC), but attempts to treat pre-term infants with RDS with aerosolized DPPC were completely unsuccessful.8–10 It is now clear that DPPC alone is a biophysically inadequate lung surfactant, and that the aerosol delivery methods used also delivered very little DPPC to the alveoli. This was not recognized at the time, and the failure of nebulized DPPC surfactant therapy led many to return to the previous clinical hypothesis of pulmonary ischemia as the cause of RDS.10 This misconception delayed further efforts to develop surfactant therapy for well over a decade. Acceptance that surfactant-deficiency was responsible for RDS did not occur until after Enhorning and colleagues11,12 demonstrated in 1973 that tracheal instillation of active whole surfactant from adult animals into pre-term rabbit pups could restore normal lung function. Even then, several more years of supportive animal studies were needed before Fujiwara et al first demonstrated the therapeutic value of exogenous surfactant in human infants with RDS in 1980.13
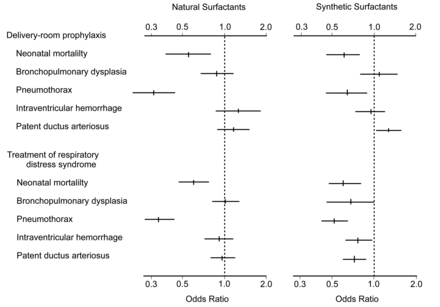
Multiple clinical trials in pre-term infants were conducted in the 1980s, following Fujiwara et al's initial successful study.5,14,15 These trials demonstrated unequivocally that exogenous surfactant improved survival and decreased morbidity in RDS (Fig. 1). Indeed, in the first year after the FDA approved surfactant therapy in the United States there was a notable decrease in neonatal mortality, primarily related to improved survival of pre-term infants.14 Surfactant therapy is now the standard of care for the prevention and treatment of RDS. As described below, the most active surfactant drugs currently approved for treatment of RDS are animal-derived preparations that contain surfactant lipids and one or more of the active hydrophobic surfactant proteins (SP-B or SP-C). In addition, several synthetic exogenous surfactants are under clinical study and laboratory research.16–24
Odds ratios and 95% confidence intervals from meta-analyses of clinical trials that found benefits from exogenous surfactant therapy for preventing or treating infant respiratory distress syndrome (RDS). Eight trials (930 infants) administered animal-derived surfactant prophylactically in the delivery room, 12 trials (1,451 infants) administered animal-derived surfactant in the intensive care nursery to treat RDS, 7 trials (1,492 infants) administered synthetic surfactant prophylactically in the delivery room, and 5 trials (2,126 infants) administered synthetic surfactant in the intensive care nursery to treat RDS. Neonatal mortality was defined as death from any cause before the 29th day of life. Bronchopulmonary dysplasia was defined as the need for oxygen, with or without assisted ventilation, together with typical radiographic changes on the 28th day of life. Intraventricular hemorrhage was defined as severe hemorrhage, categorized as either grade III (extensive intraventricular hemorrhage with ventricular enlargement) or grade IV (intraparenchymal hemorrhage). (Adapted from Reference 14, with permission.)
Pharmaceutical Surfactants
Before delving too deeply into the potential merits of surfactant therapy in ALI/ARDS, it is important to recognize that there are several different types of pharmaceutical surfactants, and their differences may have important implications for their efficacy. Endogenous pulmonary surfactant is a complex mixture of lipids (primarily phospholipids) and specific apoproteins that is highly conserved across mammalian species. The degree of resemblance of pharmaceutical surfactants to native surfactant is highly variable, and clinical preparations can be divided conceptually into 3 groups5,16,18,25:
Organic solvent extracts of lavaged lung surfactant from animals (bovactant, bovine lung extract surfactant, calfactant)
Organic solvent extracts of processed animal lung tissue, with or without additional synthetic additives (poractant alfa, beractant or surfactant-TA)
Synthetic preparations that do not contain surfactant material from animal lungs (ALEC, colfosceril palmitate, lucinactant, recombinant protein C surfactant)
Surfactants in categories I and II are sometimes classified as natural surfactants, and have the closest compositional analogy to endogenous surfactant. Category I surfactants are obtained directly from alveolar lavage fluid, and in principle contain all the surfactant phospholipids plus the hydrophobic surfactant proteins SP-B and SP-C in close approximation to the natural ratio (the hydrophilic surfactant proteins SP-A and SP-D are removed by organic solvent extraction in all category I and category II surfactants). Surfactant preparations in category II also contain surfactant phospholipids and one or both of the hydrophobic surfactant proteins, but in addition may contain cellular lipids and/or fragments of cellular proteins because they are derived from processed lung tissue. Moreover, during processing, the content of functionally important surfactant proteins can be affected (eg, SP-B is reduced to a very low level in beractant during its preparation from bovine lungs26–29).
Category III synthetic lung surfactants have conceptual advantages in purity, reproducibility, manufacturing quality control efficiency, and scale-up economy compared to animal-derived surfactants. They are also free from the risk of prion transmission, and are not subject to cultural and religious issues that can affect bovine or porcine surfactants. However, it has proved to be challenging to bioengineer fully synthetic surfactants having high activity equivalent to native surfactant. Two early protein-free synthetic surfactants (ALEC and colfosceril palmitate) are no longer used clinically because their activity is substantially less than existing animal-derived surfactants.24,25,27 Two synthetic surfactants currently under clinical study are lucinactant (KL4 surfactant) and recombinant protein C surfactant (recombinant SP-C surfactant). However, the 21-residue KL4 peptide in lucinactant only roughly approximates the overall ratio of hydrophobic to charged residues in SP-B, without any direct sequence analogy to the native protein. Also, recombinant protein C surfactant contains modified recombinant SP-C but no SP-B component.
SP-B appears to be the more important of the 2 hydrophobic surfactant proteins. Extensive laboratory studies have documented that SP-B is more active than SP-C in interacting biophysically with lipids in lung surfactant activity,29–38 and supplementation with SP-B or synthetic SP-B peptides increases the activity of surfactants containing only SP-C in animal models.26,29,39 Knock-out mice with isolated SP-B deficiency die shortly after birth, of respiratory failure,40 and human infants with SP-B mutations do not survive beyond the first days of life without surfactant replacement (and ultimately lung transplantation).28,41–43 An elegant series of experiments by Ikegami et al44 using a conditional knock-out mouse model demonstrated that adult mice rendered acutely deficient in SP-B develop severe respiratory distress with evidence of surfactant dysfunction and pulmonary inflammation. Mice left SP-B deficient died with pathology resembling ARDS, but the abnormalities were reversed and the mice survived if SP-B synthesis was restored. Interestingly, these mice maintained normal levels of the SP-C protein during study.44
Recent advances in molecular bioengineering and peptide chemistry also provide the potential to design new even more active synthetic lung surfactants, and several approaches are being studied.19,20,24,45 These include fully synthetic surfactants bioengineered to contain peptides that incorporate functionally crucial structural regions in human SP-B, such as the highly active super mini-B peptide recently reported by Waring and colleagues.22 New synthetic surfactants can also contain peptide components that incorporate active regions of other human surfactant apoproteins in combination with SP-B peptides.19 Synthetic exogenous surfactants containing super mini-B or related peptides can also include novel lipids designed to have further beneficial molecular properties such as phospholipase-resistance. One particularly active synthetic lipid analog of this kind is DEPN-8, a phospholipase-resistant diether lipid developed by Notter and co-workers.19,21,23,46,47 Synthetic surfactants containing DEPN-8 or other phospholipase-resistant lipids plus active SP-B peptides have the potential for particular utility in ALI/ARDS,19,21,23,48–50 where these lytic enzymes can be elaborated in high concentrations during the inflammatory response in injured lungs.51–57
Regardless of category (animal-derived or synthetic), the requirements for an effective therapeutic surfactant in ALI/ARDS are more stringent than is the case for RDS. To treat severe acute inflammatory lung injury, exogenous surfactant must have the greatest possible activity and resistance to inhibition or inactivation. Compositional differences among pharmaceutical surfactants are a major factor in clinical efficacy. The lack of efficacy of surfactant therapy in several randomized clinical trials in adults with ALI/ARDS may relate at least in part to inadequacies of the specific surfactant drugs used. For example, the protein-free exogenous surfactant colfosceril palmitate has some activity in treating RDS,58–61 but it is lower than that of apoprotein-containing animal surfactants such as calfactant,62,63 and colfosceril palmitate has no clinical benefits in adults with ARDS.64 Similarly, beractant has minimal benefits in adults with sepsis-induced ARDS,65 which correlates with its very low content of active SP-B. However, beractant still has substantial activity because of its content of SP-C,29 and it is efficacious in treating premature infants with RDS and term infants with acute respiratory failure from meconium aspiration lung injury, as detailed below.66–69
The Biologic Plausibility of Surfactant Therapy in ALI/ARDS
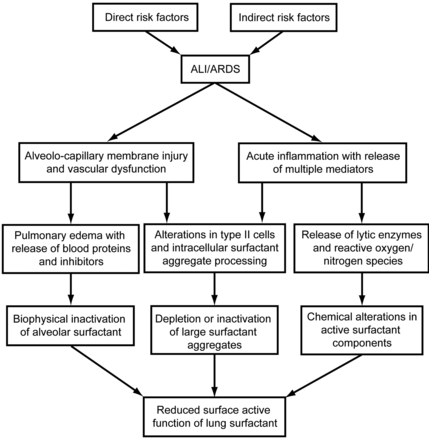
Surfactant replacement therapy in RDS makes intuitive sense, because surfactant is deficient in the premature lung and exogenous surfactant directly corrects this deficiency. The situation in ALI/ARDS, however, is more complex. Unlike RDS, surfactant dysfunction rather than deficiency is the more important contributor to ALI/ARDS pathophysiology. In ALI/ARDS an initially functional pulmonary surfactant system becomes collateral damage to whatever primary process injures the lung, irrespective of whether the injury originates on the alveolar side (direct lung injury such as from aspiration, pulmonary infection, oxygen or toxicity) or from the vascular side (indirect or extra-pulmonary lung injury such as from sepsis, shock, pancreatitis, burn injury, or multiple blood transfusions). During both direct and indirect lung injury, initially active surfactant can be rendered dysfunctional by a number of mechanisms (Fig. 2). Specific mechanisms of surfactant dysfunction in lung injury include5,70–72:
Inhibition of surfactant biophysical function by plasma proteins27,35,73–79 or other blood components such as fatty acids,79–84 that can leak into the alveolar spaces as a result of alveolo-capillary membrane injury and decreased barrier integrity
Alterations in alveolar surfactant aggregates, whereby the most active large aggregate forms of surfactant are reduced in activity and/or percent content, while less active small aggregate forms of surfactant become more prevalent72,80,85–93
Biophysical inhibition and/or chemical alteration of components in the alveolar surfactant film induced by cell membrane lipids,75,79,83,94–96 meconium,97 or other substances present during the innate pulmonary inflammatory response, such as proteases,98 phospholipases,23,99,100 or reactive oxygen/nitrogen species84,101–103
Altered synthesis, secretion, or composition of active surfactant due to injury-induced changes in alveolar type II pneumocytes, which are the primary cells of lung surfactant metabolism104–107
Pathways that can contribute to surfactant dysfunction in acute inflammatory pulmonary injury (acute lung injury [ALI] and acute respiratory distress syndrome [ARDS]). Initiators of lung injury can act either from the alveolar side (direct lung injury) or from the vascular side (indirect or extra-pulmonary lung injury). Both the direct and indirect etiologies induce pulmonary inflammation, alveolo-capillary membrane injury, permeability edema, and reactive vasoconstriction or other vascular dysfunction. In conjunction with this complex pathology, surfactant dysfunction can occur via the multiple mechanisms shown in the figure and described in the text. The resulting loss of surface-active function contributes to decreased lung volume, decreased compliance, severe ventilation-perfusion mismatching, and acute respiratory failure. (Data from References 5 and 72.)
All of the above mechanistic pathways of surfactant dysfunction are potentially present in the complex pathology of ALI/ARDS lung injury, and abnormalities in surfactant activity, large aggregate content, or composition have been well documented in bronchoalveolar lavage from patients with ALI/ARDS.92,93,108–113 Regardless of mechanism, the practical consequences of surfactant dysfunction in ALI/ARDS are not dissimilar to those in RDS. As a result of decreased surfactant activity, the lungs become less compliant, develop progressive loss of aerated volume, and manifest a worsening ventilation/perfusion mismatch. Hypoxia, respiratory failure, and the need for respiratory support ensue. Additionally, ALI/ARDS involves pulmonary inflammation and vascular dysfunction that can substantially impact overall patient outcomes and response to therapy. Systemic inflammation and multi-organ (extra-pulmonary) pathology are particularly prominent in indirect forms of ALI/ARDS. The complex pathophysiology of ALI/ARDS is reviewed elsewhere.18,114–121
It is important for therapeutic applications to recognize that lung injury in ALI/ARDS is a dynamic process. Initial acute inflammation and surfactant dysfunction can evolve to a state where pulmonary pathology is heterogeneous and includes important aspects of chronic or sub-chronic inflammation, fibroproliferative repair, vascular remodeling, and so on. Within several days following an acute pulmonary injury, type II pneumocytes also undergo proliferation and can subsequently dedifferentiate to type I cells (type II pneumocytes are stem cells for the alveolar epithelium in addition to their surfactant metabolic roles). Early interventions with exogenous surfactant therapy are likely to be most effective before type II cell changes and other chronic or sub-chronic aspects of fibroproliferative pathology are prominent in the lungs. The evolution of lung injury may also be exacerbated by positive-pressure ventilation—so-called ventilator-induced lung injury (VILI)—further complicating the pathophysiology.122–125 A major benefit of early exogenous surfactant may be to minimize or ameliorate VILI. Improved compliance and better aeration at lower pressures and volumes with early surfactant administration may prevent a vicious circle of ventilator-induced injury that leads to increasingly and more injurious levels of ventilator support, leading to further iatrogenic lung injury.
Additional Surfactant Can Overcome Surfactant Inhibition/Dysfunction
Because lung surfactant acts on respiratory mechanics by virtue of its surface-active properties, measurements of these surface-active properties in vitro have direct physiological importance and provide highly relevant insights about the rationale for surfactant-based therapies. Multiple in vitro studies have shown that pulmonary surfactant's ability to lower surface tension is impaired by exposure to albumin, hemoglobin, lyso-phospholipids, fatty acids, and other substances that leak into the alveolar space in association with injury to the alveolar-capillary membrane, as described in the preceding section.27,35,73–84 Importantly, those studies also document that inhibitor-induced inactivation can be overcome by raising surfactant concentration, offering a plausible mechanism whereby exogenous surfactant administration might improve lung function after acute injury. The ability of exogenous surfactants to overcome inhibition in vitro depends not only on surfactant concentration, but also on their content of essential apoproteins, particularly SP-B. Exogenous surfactants with higher contents of SP-B generally have greater activity and better inhibition resistance than those with little or no SP-B.5 This is particularly relevant clinically, because the 3 major surfactant drugs studied to date in controlled trials in adults with ARDS contain either no SP-B (colfosceril palmitate and recombinant protein C surfactant)64,126,127 or minimal SP-B (beractant).65
Animal Studies of Surfactant
Although the existence of surfactant dysfunction in ALI/ARDS and the fact that it can be overcome in vitro by increasing the concentration of active surfactant provide a conceptual rationale for surfactant replacement, evidence from animal studies is also required to support human therapy. Animal model studies of ALI/ARDS have some practical limitations, including:
They largely evaluate short-term outcomes such as oxygenation improvement or acute changes in lung histology.
The injury initiators and species used are diverse and complicate comparisons.
Some models, such as saline lavage (one of the most commonly used ALI/ARDS models), resemble RDS more than ALI/ARDS.
It is difficult to assess potentially crucial clinical factors such as VILI in short-term animal experiments.
Despite these shortcomings, animal models are an invaluable complement to clinical trials in humans. Animal models of ALI/ARDS can be divided into direct and indirect injury models. In analogy with clinical ALI/ARDS, direct models employ injuries originating on the alveolar side of the alveolar-capillary membrane (eg, animals given pneumonia, acid aspiration, intratracheal toxin administration, hyperoxic exposure). Animals with isolated pulmonary contusion from focused blunt closed-chest trauma are also conceptually in the direct injury category. Indirect ALI/ARDS models involve systemic injuries originating on the capillary side of the alveolar-capillary membrane (eg, animals given sepsis, intravenous oleic acid, or other systemic toxin administration). The response to exogenous surfactant in direct lung-injury models has generally been positive, whereas treatment responses are less favorable in indirect injury models.5,71,72
One classic direct ALI/ARDS lung-injury model involves mechanically ventilated animals undergoing repeated lung lavage with warmed saline until a level of hypoxia consistent with ARDS results. This model has been studied in several mammalian species, and the response to instilled active exogenous surfactant has been consistently positive.22,39,128–137 However, lung lavage primarily induces surfactant deficiency resembling RDS rather than ALI/ARDS, although superimposed injury from in vivo lavage and mechanical ventilation is also present. Other animal models of direct pulmonary ALI/ARDS have also responded favorably to active exogenous surfactant. Experiments in 2 models of viral infection, one with Sendai virus in mice,138 and another with influenza A virus,139 show improved oxygenation and lung histology with exogenous surfactant.
Exogenous surfactant therapy is similarly beneficial in animals with pneumonia from Pneumocystis carinii,140 group B streptococcus,141 and acid aspiration pneumonitis.142–145 Direct lung injury from tracheally instilled lipopolysaccharide in animals also responds favorably to exogenous surfactant administration,146,147 and there was significant favorable response to exogenous surfactant in animals with acute hyperoxic injury relevant for ALI/ARDS.148,149
In contrast, animal models of indirect lung injury generally respond less well to surfactant administration. Injury from oleic acid infusion via the pulmonary artery shows almost no improvement with surfactant,150 although many would argue that this is a lethal injury with substantial cellular disruption151 that is unlikely to respond to any therapy. A porcine sepsis model that used intravenous lipopolysaccharide infusion showed some oxygenation improvement following surfactant administration,152,153 but the response was less striking than in the surfactant-treated animals injured with intratracheal lipopolysaccharide above.146,147 It is notable that the different responses to surfactant between direct and indirect lung injury in animals resemble the response pattern seen in humans; analysis of 2 trials found that direct pulmonary ALI/ARDS is impacted most beneficially by surfactant therapy.127,154
In addition to evaluating efficacy, animal models can also be very useful in evaluating other aspects of surfactant therapy, such as optimizing delivery protocols, doses, and the timing of therapy. Studies of this kind may be descriptive from a scientific perspective, but they can have great practical benefit in optimizing delivery, distribution, and dosing-related aspects of surfactant therapy. However, animal work to date has not been particularly extensive or systematic in investigating these phenomena. One delivery-related area that has received attention in animal studies involves surfactant aerosolization. Multiple studies have reported respiratory improvement with surfactant aerosol in animals with surfactant deficiency or dysfunction,131,135,153,155–163 although other aerosol studies have been less positive.164–167 Several animal studies have directly compared aerosolization to tracheal instillation, and reported that aerosolization can have equal or greater effectiveness,131,155,159 whereas others have found that instillation is more effective than aerosolization.131,165,168 An example illustrating the need for further clarity on this issue is the study by Lewis et al.131 In lung-lavaged sheep, exogenous bovine lung extract surfactant was effective when instilled but ineffective when aerosolized, whereas beractant in the same animal model was more effective when aerosolized than when instilled. Clinical studies with aerosolized surfactant have in general not had impressive results. A small pilot study by Jorch et al169 reported that aerosolized bovactant improved the alveolar-arterial oxygen difference in older pre-term infants (28–35 weeks gestation), but studies with other aerosolized surfactants in pre-term infants found no respiratory benefits,170–172 despite the known efficacy of instilled exogenous surfactant in that patient population. In addition, a large controlled study by Anzueto et al,64 in which they administered aerosolized colfosceril palmitate to adults with ALI/ARDS, was also negative, as discussed in more detail below.
If it is possible to perfect aerosol delivery technology for lung surfactants, this is clearly a conceptually attractive alternative to instillation for clinical application. Aerosol delivery might avoid the transient endotracheal tube (ETT) obstruction and resultant hypoxia and hypotension seen with bolus instillation. However, delivering aerosolized surfactant in sufficient quantity and making it distribute evenly throughout the alveoli of injured lungs is non-trivial. Aerosol enters only ventilated lung units.157,158,173 Aerosol delivery to the alveoli in normal lungs is maximal in the particle-size range 0.5–2.0 μm, and it is possible to generate a stable surfactant aerosol with that particle size range from aqueous or powdered surfactant.135,174,175 A recent study by Ruppert et al135 demonstrated the ability to deliver substantial amounts of a powder recombinant protein C surfactant aerosol with a new aerosol generator to improve oxygenation and compliance in lung-lavaged rabbits. However, whether surfactant aerosolization can be accomplished in a sufficiently effective and efficient manner to replace instillation requires further and more detailed direct comparisons in animals and subsequently in human trials.
Animal studies have also investigated issues relevant to the preferred timing of surfactant therapy in ALI/ARDS. Bjorklund et al176 showed in lambs, and Hillman et al177 in sheep, that positive-pressure ventilation of the surfactant-deficient lung can result in substantial lung injury within minutes, which supports the view that early surfactant therapy is crucial. In addition, both animal178,179 and human studies180,181 have demonstrated that prophylactic administration of surfactant is more effective than later rescue surfactant delivery because of better surfactant distribution and avoidance of VILI. Whether the same is true in ALI/ARDS is unknown, but by analogy it would seem likely that early surfactant treatment of ALI/ARDS would be more effective.
Other aspects of surfactant therapy that have been explored in animal studies include the volume and dose of surfactant,182 rapidity of administration,183 and method of respiratory support during surfactant administration.163,184 As a general rule, a larger instillation volume and/or higher dose results in more uniform distribution.182 Unfortunately, a larger instilled volume can also cause severe hypoxia and hypotension, due to transient ETT obstruction.154,185 The same trade-off is true for giving surfactant as a rapid bolus, as opposed to over a longer period; that is, a more homogeneous distribution is achieved with a rapid bolus, but this also risks obstructing the ETT. Using an alveolar recruitment maneuver (eg, manual bagging or a larger tidal volume) during instillation may facilitate surfactant distribution and reduce the degree or duration of hypoxia and hypotension. Studies by Krause et al136,137 in young rabbits and piglets with lung injury induced by in vivo lavage found that several methods of volume recruitment (increased ventilator peak inspiratory pressure, tidal volume, and/or PEEP) at the time of instillation significantly improved the pulmonary efficacy of surfactant therapy.
In summary, animal studies have helped to elucidate several aspects of surfactant efficacy and administration, and remain indispensible for investigating surfactant therapy and its optimization going forward. However, animal studies typically examine a relatively brief experimental timescale, and necessarily involve the use of non-human species. Questions of safety and clinical efficacy ultimately require studies with human patients.
Human Studies
A number of uncontrolled and controlled studies have documented clinical benefit from exogenous surfactant replacement therapy in term infants, children, and adults with acute respiratory failure (ALI/ARDS) 66–69,154,186–200 (Table 2). Of the 19 positive studies listed in Table 2, 9 were controlled trials67–69,154,192,197–200 and 10 were uncontrolled treatment trials, in which the risk of selection bias is inherent. An additional 4 positive case series found clinical improvement from surfactant therapy but are not included in Table 2.201–204 Figure 3 describes the clinical course of a representative child who received surfactant (calfactant) after near-drowning.205 Near-drowning washes out pulmonary surfactant and is often accompanied by hypoxia and aspiration of gastric contents, which may lead to permeability lung injury. Thus, it can involve both surfactant deficiency and dysfunction. Radiographic changes in lung aeration are clear and are reflected in the dramatic oxygenation improvement associated with calfactant administration.
Selected Controlled and Uncontrolled Clinical Studies That Reported Significant Benefits From Exogenous Surfactant Therapy in ALI/ARDS
Clinical course of a child before and after calfactant administration. HFOV = high-frequency oscillatory ventilation. INO = inhaled nitric oxide. CT = chest tube. (Adapted from Reference 205.)
Despite the relatively small size and mixed nature of the studies in Table 2, the sum of their findings shows that there are a number of specific indications where the evidence for the use and efficacy of exogenous surfactant therapy in acute pulmonary injury is compelling. One of the best-studied applications of surfactant therapy for an indication other than RDS is in full-term infants with meconium aspiration syndrome.66–69,196 Meconium is a thick, tarry mixture of bile acids and mucous glycoproteins that fills the fetal colon during gestation, and prenatal defecation and gasping episodes associated with maternal/fetal stress can lead to meconium aspiration at or prior to birth. Meconium mechanically obstructs airways, causes inflammation,206 and inhibits the biophysical activity of lung surfactant.97,207 Auten et al,196 Khammash et al,66 and Findlay et al67 have all reported significant lung-function improvement following exogenous surfactant administration (calfactant, beractant) to infants with meconium aspiration syndrome. The randomized controlled study by Findlay et al67 also found significant reductions in the incidence of pneumothorax, duration of mechanical ventilation and oxygen therapy, time of hospitalization, and extracorporeal membrane oxygenation (ECMO) requirement in 20 term infants with meconium aspiration syndrome treated with beractant, compared to a similar number of controls. Lotze et al68 also reported favorable results with beractant in a controlled trial in term infants referred for ECMO due to severe respiratory failure (meconium aspiration was a prevalent diagnosis). A subsequent larger multicenter controlled trial with 328 term infants similarly reported significant improvements in respiratory status and decreased need for ECMO following surfactant treatment.69 Exogenous surfactant is now routinely used in many institutions to treat neonates with meconium aspiration syndrome, as well as infants with respiratory failure from pneumonia. Clinical studies documenting the efficacy of surfactant therapy in pneumonia are less extensive than for meconium aspiration syndrome, but those that are available have been positive.196–198 An initial uncontrolled study by Auten et al196 reported significant improvements in oxygenation in term infants with pneumonia following instillation of calfactant (calf lung surfactant extract [CLSE]). Also, Luchetti et al197,198 reported both improved oxygenation and shortened duration of ventilation following treatment with porcine surfactant (poractant alfa, 50 mg/kg) in 2 small controlled but unblinded studies of infants with RSV bronchiolitis.
Experience with clinical surfactant therapy in adults with ALI/ARDS has been much less encouraging than in younger patients. In a large controlled trial, Anzueto et al64 administered nebulized colfosceril palmitate or placebo to 725 adults with ARDS secondary to sepsis and found no oxygenation improvement and no effect on morbidity or mortality. However, that study was flawed by the facts that colfosceril palmitate is a poor surfactant, and the aerosol technology they used delivered very little colfosceril palmitate to the alveoli. Additionally, it appears now, from both animal and human data, that surfactant therapy is inherently less effective in indirect lung injury such as sepsis, compared to direct pulmonary ALI/ARDS. Several other controlled clinical trials of surfactant therapy in adults have also reported disappointing results. Gregory et al65 found some oxygenation improvement for a subgroup of patients with sepsis-induced ALI/ARDS who received intermediate-size doses of beractant (100 mg/kg), but no oxygenation improvement in other treated groups, and no long-term benefits in the overall 43 surfactant-treated patients studied.
Spragg et al,127 using synthetic SP-C surfactant (recombinant protein C surfactant), also reported improved oxygenation but no long-term benefits, relative to placebo, in adults with ARDS. On post hoc analysis, however, surfactant-treated subjects with direct lung injury appeared to have better survival, so Spragg et al carried out a similarly designed randomized controlled trial focused on direct lung injury. Unfortunately, that study was stopped for futility after an interim analysis at 800 subjects (personal communication, Roger Spragg, Division of Pulmonary and Critical Care Medicine, University of California School of Medicine, San Diego, California, 2010). Finally, a study by Kesecioglu et al,185 with a porcine surfactant called HL-10, was also stopped at an interim analysis because of a potential harmful effect. They instilled surfactant in 2 large boluses and saw a significant incidence of peri-dosing hypoxia and hypotension (> 60%). There was also a suggestion of higher mortality in the surfactant group at the 6-month follow-up, which was not seen at 3 months, although there is no known plausible mechanism for that higher mortality.
Exogenous surfactant therapy was successfully used in a randomized controlled study by Amital et al,200 in 42 adult patients following lung transplant. The calfactant-treated patients had better oxygenation, less post-graft dysfunction, shorter ICU stay, and better lung function at one month. That study followed multiple uncontrolled case studies that also reported clinical improvements from exogenous surfactant therapy in adults and children after lung transplantation.201–203
Controlled studies of surfactant therapy in children with ALI/ARDS have been more encouraging than those in adults. A randomized but unblinded trial by Willson et al192 in 42 children with ALI/ARDS found that those who received calfactant (70 mg/kg) had immediate improvement in oxygenation and fewer ventilator days and days in intensive care. That trial followed an initial uncontrolled treatment study by the same group, which found improved oxygenation in 24 children (ages 0.1–16 y) with ALI/ARDS treated with instilled calfactant.191
A controlled study by Moller et al199 also reported that children with ARDS showed immediate oxygenation improvement and less need for rescue therapy following treatment with beractant, but the trial was underpowered to assess more definitive longer-term outcomes. A larger and more recent blinded controlled study in 2005 by Willson et al,154 in patients up to age 21 years with ALI/ARDS, found that treatment with calfactant, relative to placebo, was associated with immediate oxygenation benefit and a significant survival advantage (Table 3). In a post hoc analysis, the benefits of surfactant therapy were confined to the 98-patient subgroup with direct pulmonary ALI/ARDS (Table 4), which prompted a larger prospective trial in both adults and children with direct lung injury: the Calfactant for Direct Acute Respiratory Distress Syndrome (CARDS) trial (http://clinicaltrials.gov/ct2/show/NCT00682500), which was a prospective, masked randomized controlled trial of calfactant versus placebo. The study was carried out in more than 30 centers in the United States, Canada, Korea, Israel, Australia, and New Zealand. The data analysis is incomplete, but, unfortunately, both the adult and pediatric arms were stopped because of futility. The reasons for the failure of this trial after the success of the previous trials of Willson et al154,192 remain to be elucidated.
Clinical Outcomes in the 2005 Study by Willson et al154
Efficacy of Exogenous Surfactant in Direct and Indirect Lung Injury in the Controlled 2005 Study by Willson et al154 in Patients Up To 21 Years Old With ALI/ARDS
Summary and the Future of Surfactant Therapy
Exogenous surfactant has a well established place in the prevention and treatment of infant RDS. Efficacy in other types of ALI in neonates (eg, meconium aspiration, neonatal pneumonia) has also been studied in controlled and uncontrolled trials, and surfactant treatment for those conditions has become fairly routine. In lung injury beyond the neonatal period, however, evidence for the efficacy of surfactant therapy is less extensive, although several controlled studies in children and older adolescents with ALI/ARDS have been encouraging.154,192 Evidence for surfactant therapy in adults with ALI/ARDS is much less compelling, and remains a work in progress.
Many factors can contribute to inconsistency in the results about surfactant therapy in ALI/ARDS. The complexity of ALI/ARDS itself undoubtedly plays a large role, as it is not a single disease but, rather, the end result of many different types of acute pulmonary injury. The more favorable response in patients with direct versus indirect lung injury found by Spragg et al127 and Willson et al154 illustrates this point. Timing of surfactant administration in ALI/ARDS may also be very important for efficacy, particularly if a major benefit of the therapy is to prevent or ameliorate VILI, which would require early use of surfactant.
The effectiveness of surfactant delivery due to differences in drug volume, drug viscosity, delivery rate, or delivery method (instillation vs aerosolization) may also be of great importance for surfactant therapy in ALI/ARDS. Pulmonary pathology in inflammatory injury is severe and heterogeneous,208–211 and getting exogenous surfactant into injured lung areas in a concentration sufficient to overcome inhibition by inflammatory and other substances is a challenge. How the lung is ventilated during and immediately after surfactant delivery can substantially impact drug distribution. The recruitment of alveoli by bagging or other recruitment maneuvers during surfactant drug delivery may open otherwise inaccessible areas of injured lung, and these may then remain open if exogenous surfactant has reached them effectively.136,137 Also, if surfactant delivery via aerosolization can be perfected, it might avoid the transient hypoxia, hypertension, and ETT obstruction associated with instillation. However, studies have shown that, despite the drawbacks, instillation benefits patients with several forms of ARDS.
Another major variable in surfactant therapy is the specific composition and activity of the exogenous surfactant drug used. A large number of studies have identified significant differences in activity between surfactant drugs at the laboratory level.5,16,18,72 Differences in exogenous surfactant activity are likely to be more crucial in treating ALI/ARDS, compared to RDS. Successful treatment of ALI/ARDS requires the most robust surfactants because of the need to resist inhibition by substances that leak into the alveolar space as a result of permeability injury. In general, surfactants with an adequate percentage of SP-B appear to be the most resistant to inhibition. It is promising that new synthetic surfactants based on active SP-B peptides are currently being developed, including super mini-B surfactant.22 New synthetic lung surfactants under development can also be formulated to contain novel active lipids that resist degradation by phospholipases in injured lungs.19,23,48
A final factor not emphasized in this article is the potential of combination therapies in ALI/ARDS. The pathophysiology of ALI/ARDS is complex and multifaceted, and single agents may not be sufficient to achieve the most substantial benefits to long-term patient outcomes. The use of exogenous surfactant in conjunction with other modalities to simultaneously attack different aspects of the pathophysiology of ALI/ARDS may prove to be synergistic—not unlike the use of combination chemotherapy for various types of cancer. As one example, if surfactant can improve the distribution of ventilation in the lung, simultaneous use of nitric oxide as a synergistic agent may increase perfusion to the newly ventilated lung units. The potential use of other pharmacologic agents, along with gentle low-tidal-volume ventilation and careful fluid management, in combination with surfactant is also possible. Detailed discussion of combination therapy approaches for ALI/ARDS are reviewed elsewhere.18,212
In summary, it is likely that surfactant will find a place in our armamentarium for the treatment of ALI/ARDS when some of the above issues are clarified. As the famous philosopher Yogi Berra so eloquently stated, “Predictions are difficult, particularly about the future.” Nonetheless, surfactant therapy has in fact already been shown to be beneficial in several lung-injury applications in term infants and children, in addition to its life-saving use in pre-term infants with RDS. The jury is still out with respect to the use of surfactant therapy in adults with ALI/ARDS, and several negative studies clearly do exist. However, the perspective of history shows that the first trials of surfactant therapy in RDS were unsuccessful due to the use of ineffective surfactants and delivery methods, and it was more than 2 decades after surfactant deficiency was first suggested to be the cause of RDS7 before Fujiwara et al13 demonstrated successful treatment of premature infants with exogenous surfactant. Lung injuries leading to clinical ALI/ARDS clearly comprise a more complicated and diverse set of conditions than RDS, and finding the most successful surfactant interventions, delivery methods, and possible synergistic combination therapies may simply require more time.
Discussion
Willson:
They used ventilation protocols, but I don't recall the details of them, and only the last two studies used low VT. Roger Spragg has always been involved with the Acute Respiratory Distress Syndrome Network, and his studies have used low VT. Lachmann's group also used a ventilation protocol that they said was modeled after the Acute Respiratory Distress Syndrome Network low-VT strategy. So, other than the first study, I think yes, they were fairly comparable in terms of how they were managed.
Curley:
Thank you for all of the work you've done over the years. You've been a pioneer and a colleague to many who've walked behind you. You are always there with good suggestions and are very generous with your work.
I think surfactant ought to be used in certain subpopulations You can't deny some of these cases. Which of the diagnoses are more responsive to surfactant?
Willson:
The devil is in the details. Distribution is a big issue. We have to work out the details regarding administration. The data are very sparse. What volume? What dose? How often? How exactly is it given? The difference between our 3 previous studies1–3 and this study (Calfactant for direct acute respiratory distress syndrome [CARDS] trial. http://clinicaltrials.gov/ct2/show/NCT00682500) relate to how it was given—not so much the patient population.
The first question I want to answer is how do we best administer it? After that, the data that we have say that it is most helpful in direct lung injury. In my own experience—and I do give it off-label when we're not involved in studies—I've seen kids in whom surfactant is like a resurrection. The very first kid I gave it to I thought was going to be on ECMO by the evening, and he was extubated 2 days later. And I've also had kids in whom it did absolutely nothing.
It's possible that there are differences in concentration, strength, et cetera, because it's a biological product. It's a very complex issue. But some of the details we've talked about are important, and I think that's the first thing. Once we figure out if there are differences in how it's distributed, depending on how you give it, then the next thing will be to look at the patient populations.
Curley:
Maybe it's in the biological processes, and we just don't know enough about the processes that we're administering the product in?
Willson:
There's no question about that. There are different types of surfactant, and it is not a simple substance.There are multiple phospholipids, neutral lipids, and surfactant proteins, all of which have to come together to be effective, and that may be one of the problems with aerosolization.
Wiswell:
Doug, I'm intrigued by the concept of administering dilute surfactant via lung lavage. I was involved with a study in the late 1990s in adults, in which surfactant was delivered via bronchoscopy to the individual pulmonary segments.1 We had some glimmers of success, but the follow-up trial fell through. I was subsequently involved in a small trial in which we directly lavaged the lungs, via the ETT, of neonates with meconium aspiration syndrome.2 We also had some glimmers there.
We subsequently performed a larger trial in meconium aspiration syndrome with this technique. Unfortunately, the pharmaceutical company sponsoring that trial has kept all the data under wraps, as they concentrated on the RDS population in their attempt to get FDA approval of their surfactant. Recently the Australia/New Zealand study3 used a similar approach in babies with meconium aspiration syndrome, lavaging the lungs with dilute surfactant. The lavaged group had lower mortality and were less likely to need ECMO.
Thus, I'm wondering if with certain disorders the lavage approach may be a better way to administer surfactant. When I think of ALI/ARDS or meconium aspiration syndrome, I think of the crap inside the lungs, including inflammatory products and other material that deactivates surfactant. It seems logical to wash out that debris and leave behind some functioning surfactant, if we can do it without harming the patient. Adults are bigger, so you can bronchoscope them easier than you can kids. On the other hand, one can lavage a baby directly via the ETT a lot easier than you can a 4-year-old or 8-year-old. Any thoughts on administration?
Willson:
There have been some uncontrolled studies, and it does make intuitive sense, particularly if distribution is a major problem: it makes intuitive sense to deliver it via bronchoscopy. From a practical point of view it would require a lot more effort to do, so I fear that study will never be done. The study you're referring to had about 90 adults, which for an adult study is small.1 One important problem with any clinical study in children is that the standard is about a 10% mortality, and that's going to be hard to beat, particularly the morbidity associated with just intubating, general anesthesia, and bronchoscopy in a small child or baby. I think we're going to have to look at it some other way. Some of the problem with debris in the lung undoubtedly develops over time, but if you could give surfactant early down the ETT I think it might help. And it may not be so much who but when: 3 days into the disease there may already be severe VILI and severe heterogeneity in the lung, and then it's too late.
Walsh:
I want to confirm your thought about recruitment. I participated as a dosing administrator in your study, and I remember hearing the care team (who was blinded to the study drug) look at the oxygen saturation and state, “Oh, this kid got surfactant!” when I knew that patient got a sham dose. I couldn't say anything, but I remember that vividly in at least 2 patients. I think there's potentially something there. When you're talking about giving it early, how early is early? Would you consider using a laryngeal mask airway and then putting them back on noninvasive ventilation?
Willson:
Like the neonates? I think the solution would be to aerosolize it, then give via noninvasive ventilation. Unfortunately, aerosolization hasn't been successful so far. If I knew which way a kid was headed, I'm sure I wouldn't hesitate to use it early, particularly if you could effectively aerosolize it. If it were my own child, that's what I would do.
But you know, Brian, if you look at a kid admitted with pneumonia, for example, early on it is difficult to determine their clinical trajectory, so getting approval for a study to treat non-intubated children seems unlikely. I think the best we can do, since aerosolization doesn't seem to work, would be to conduct a study in which the surfactant is instilled immediately after intubation. This would allow the best possible distribution and the possibility of avoiding some of the positive-pressure-associated lung injury, and would be the best test of whether instilled surfactant is helpful.
Rubin:
Actually, surfactant has been successfully aerosolized into humans and produced a benefit. We published a COPD trial in JAMA that showed significant mobilization of secretions and improvement in pulmonary function.1 A similar study was done in persons with cystic fibrosis. So there may be different end points when used as an ambulatory therapy.
Willson:
What were the end points in that study?
Rubin:
Pulmonary function, secretion biophysical properties and trans-portability, and gas trapping as mea-sured via DLCO [diffusing capacity of the lung for carbon monoxide].
Willson:
I was not aware of those data. Which surfactant was it?
Rubin:
The study was done a long time ago. It was Exosurf. I was the senior author.
Willson:
Interesting. An incomplete surfactant to say the least. Thank you.
Curley:
Doug, in the current study (Calfactant for direct acute respiratory distress syndrome [CARDS] trial. http://clinicaltrials.gov/ct2/show/NCT00682500) what was the number of patients enrolled per site?
Willson:
In the pediatrics study I think it was 32. In the adult study there were about 60.
Curley:
And the number enrolled per site?
Willson:
Some sites, as you can imagine, only had one. Omaha Children's had 18, I think. There was a wide range of numbers
Curley:
It might be interesting to take away the first 3 or 4 patients and look at the data after that, because it is operator-dependent, and even in the activated protein-C trial the first 3 or 4 patients per site showed different outcomes than those who followed. It shows that expertise at the bedside matters.
Willson:
I appreciate the suggestion. We're trying to determine if there is a patient population that responds acutely and whether there are differences between the sites. Some of that will depend on how many different patients you enroll in the study. We're also beginning to look at the adult data and whether there are differences between the children and adults. My guess is not, but we'll see.
Footnotes
- Correspondence: Douglas F Willson MD, Department of Pediatrics, University of Virginia Children's Hospital, Charlottesville VA 22908-0386. E-mail: dfw4m{at}virginia.edu.
Dr Willson presented a version of this paper at the 47th Respiratory Care Journal Conference, “Neonatal and Pediatric Respiratory Care: What Does the Future Hold?” held November 5–7, 2010, in Scottsdale, Arizona.
Dr Willson has disclosed relationships with ONY Inc and Pneuma Pharmaceuticals.
- Copyright © 2011 by Daedalus Enterprises Inc.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.
- 31.
- 32.
- 33.
- 34.
- 35.↵
- 36.
- 37.
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.
- 50.↵
- 51.↵
- 52.
- 53.
- 54.
- 55.
- 56.
- 57.↵
- 58.↵
- 59.
- 60.
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.
- 75.↵
- 76.
- 77.
- 78.
- 79.↵
- 80.↵
- 81.
- 82.
- 83.↵
- 84.↵
- 85.↵
- 86.
- 87.
- 88.
- 89.
- 90.
- 91.
- 92.↵
- 93.↵
- 94.↵
- 95.
- 96.↵
- 97.↵
- 98.↵
- 99.↵
- 100.↵
- 101.↵
- 102.
- 103.↵
- 104.↵
- 105.
- 106.
- 107.↵
- 108.↵
- 109.
- 110.
- 111.
- 112.
- 113.↵
- 114.↵
- 115.
- 116.
- 117.
- 118.
- 119.
- 120.
- 121.↵
- 122.↵
- 123.
- 124.
- 125.↵
- 126.↵
- 127.↵
- 128.↵
- 129.
- 130.
- 131.↵
- 132.
- 133.
- 134.
- 135.↵
- 136.↵
- 137.↵
- 138.↵
- 139.↵
- 140.↵
- 141.↵
- 142.↵
- 143.
- 144.
- 145.↵
- 146.↵
- 147.↵
- 148.↵
- 149.↵
- 150.↵
- 151.↵
- 152.↵
- 153.↵
- 154.↵
- 155.↵
- 156.
- 157.↵
- 158.↵
- 159.↵
- 160.
- 161.
- 162.
- 163.↵
- 164.↵
- 165.↵
- 166.
- 167.↵
- 168.↵
- 169.↵
- 170.↵
- 171.
- 172.↵
- 173.↵
- 174.↵
- 175.↵
- 176.↵
- 177.↵
- 178.↵
- 179.↵
- 180.↵
- 181.↵
- 182.↵
- 183.↵
- 184.↵
- 185.↵
- 186.↵
- 187.
- 188.
- 189.
- 190.
- 191.↵
- 192.↵
- 193.
- 194.
- 195.
- 196.↵
- 197.↵
- 198.↵
- 199.↵
- 200.↵
- 201.↵
- 202.
- 203.↵
- 204.↵
- 205.↵
- 206.↵
- 207.↵
- 208.↵
- 209.
- 210.
- 211.↵
- 212.↵
- 1.↵