Abstract
BACKGROUND: Children with severe bronchospasm requiring mechanical ventilation may become refractory to conventional therapy. In these critically ill patients, isoflurane is an inhaled anesthetic agent available in some centers to treat bronchospasm. We hypothesized that isoflurane is safe and would lead to improved gas exchange in children with life-threatening bronchospasm refractory to conventional therapy.
METHODS: A retrospective review was conducted and included mechanically ventilated children treated with isoflurane in a quaternary pediatric ICU for life-threatening bronchospasm, from 1993 to 2007. Demographic, blood gas, ventilator, and outcome data were collected.
RESULTS: Thirty-one patients, with a mean age of 9.5 years (range 0.4–23 years) were treated with isoflurane, from 1993 to 2007. Mean time to initiation of isoflurane after intubation was 13 hours (0–120 h), and the mean maximum isoflurane dose was 1.1% (0.3–2.5%). Mean duration of isoflurane administration was 54.5 hours (range 1–181 h), with a total mean duration of mechanical ventilation of 252 hours (range 16–1,444 h). Isoflurane led to significant improvement in pH and PCO2 within 4 hours of initiation (P ≤ .001). Complications during isoflurane administration included hypotension requiring vasoactive infusions in 24 (77%), arrhythmia in 3 (10%), neurologic side effects in 3 (10%), and pneumothorax in 1 (3%) patient.
CONCLUSIONS: Isoflurane led to improvement in pH and PCO2 within 4 hours in this series of mechanically ventilated patients with life-threatening bronchospasm. The majority of patients in this series developed hypotension, but there was a low incidence of other side effects related to isoflurane administration. Isoflurane appears to be an effective therapy in patients with life-threatening bronchospasm refractory to conventional therapy. However, further investigation is warranted, given the uncertain overall impact of isoflurane in this context.
Introduction
Over 9 million children in the United States suffer from asthma, making it the most common chronic disease of childhood.1 Despite substantial public health efforts and medical advances over the past several years, the prevalence and mortality of pediatric asthma remain largely unchanged.2 Morbidities related to childhood asthma lead to more than 6 million office visits, 700,000 emergency department visits, 200,000 hospitalizations, and 200 deaths each year.2
Children who present with life-threatening episodes of bronchospasm requiring intubation and ICU admission represent a small fraction of asthma exacerbations, but the care of these patients is the source of substantial healthcare cost and considerable complications.3 The treatment of these patients with bronchospasm and acute respiratory failure who require mechanical ventilation is often challenging, and difficulty in controlling the ventilation of bronchospastic lungs is often particularly troublesome. These patients sometimes become refractory to conventional therapies, leading some centers to employ inhaled anesthetic agents to aid in achievement of adequate gas exchange in these most severely affected patients.4–11
Inhaled anesthetics have inherent bronchodilatory properties that are the pharmacologic basis for their administration as a therapeutic option for bronchospasm. In addition, the anesthetic properties of these medications provide excellent sedation for these patients to assist with facilitation of mechanical ventilation. Inhaled agents produce bronchodilation both directly, via relaxation of airway smooth muscle cells, and indirectly, through the depression of protective airway reflexes.12,13 Isolated reports of inhaled anesthetics for the treatment of refractory bronchospasm in adult patients can be found as early as the 1930s,14–16 and through the 1970s a number of investigators reported the use of halothane, enflurane, sevoflurane, or isoflurane in adult patients with asthma refractory to traditional therapies and asthmatic patients needing general anesthesia.4–7,17 This use of inhaled anesthetics in the context of bronchospasm expanded to pediatric patients in the 1980s and 1990s; however, use of these agents continues to remain limited.8–11
Isoflurane has become somewhat more prevalent than other inhaled anesthetics as a therapeutic option for refractory bronchospasm over the past decade in the pediatric ICU (PICU), primarily due to its safety profile relative to other agents.18–20 However, the use of inhaled anesthetics outside of the operating room remains relatively uncommon, due to the need for both experienced providers and appropriate equipment for the delivery and scavenging of these volatile agents. Inhaled anesthetics, like isoflurane, present substantial challenges in the PICU setting, given their volatility and potential impact on bedside providers, but in recent years, growing interest has emerged in expansion of the administration of inhaled anesthetics for a wide range of applications in the PICU.18,19 In addition, improved integration of ventilator technology and capabilities in modern anesthesia machines may allow for safer delivery of these agents in a wider range of clinical settings. We undertook this retrospective review to describe our experience with the use of isoflurane for life-threatening bronchospasm in the PICU at our institution over a 15 year period.
QUICK LOOK
Current knowledge
Isoflurane is an anesthetic agent with inherent bronchodilatory properties. Isoflurane has been used to treat life-threatening bronchospasm during mechanical ventilation, on a case by case basis.
What this paper contributes to our knowledge
Delivery of isoflurane to mechanically ventilated pediatric patients with life-threatening bronchospasm results in improved ventilation, as evidenced by a reduction in arterial carbon dioxide and increase in pH. Hypotension was a frequent complication occurring in three quarters of patients studied.
Methods
After Children's Hospital Boston institutional review board approval, the medical records of all patients with life-threatening bronchospasm and acute respiratory failure necessitating endotracheal intubation and mechanical ventilation who were treated with isoflurane, from January 1993 through December 2007, in the PICU at Children's Hospital Boston were identified from a respiratory therapy database. Records were reviewed with attention to demographic information, diagnosis, dosing, and duration of isoflurane administration. We also recorded all blood gas measurements, duration of mechanical ventilation, outcome, and need for ongoing respiratory support upon discharge. Further data were collected related to side effects attributable to isoflurane, which included hypotension (defined as the need for vasoactive medications to support blood pressure despite fluid administration following initiation of isoflurane), air leak, arrhythmia, and neurologic dysfunction (defined as any new neurologic finding or abnormality during isoflurane administration).
Following intubation, all patients were treated with pressure limited ventilation. While no ventilator guideline or protocol was used for these patients, in general, the approach to mechanical ventilation was to minimize lung injury by allowing permissive hypercapnia, while also minimizing both mean airway pressure and FIO2. Ventilatory strategies, along with all other management decisions, ultimately were made by the pediatric intensivist-led clinical team caring for the patient at the bedside. Therapies utilized in all patients included both inhaled β agonists and intravenous steroids, and most patients were also treated with a number of other adjunctive agents. These additional interventions included intravenous β agonists, inhaled ipratropium bromide, intravenous methylxanthines, intravenous magnesium sulfate, and intravenous ketamine.
Isoflurane was initiated at the discretion of the care team, based on clinical progression and trajectory. Prior to initiation of isoflurane, family and anesthetic histories were taken, and patients with a personal or family history of malignant hyperthermia or myopathy were not treated with isoflurane. Starting dose was determined by the clinical team, and there was no standard protocol for isoflurane initiation and titration, but, in general, the goal was to maintain a minimal alveolar concentration of approximately 0.5–1.0. A minimal alveolar concentration of 1.0 represents the dose sufficient to prevent movement upon surgical incision in 50% of patients. Isoflurane was administered in all cases using an isoflurane vaporizer and self-scavenging system fitted to a Servo 900C ventilator (Maquet, Bridgewater, New Jersey). An agent monitor (Datex Ohmeda Ultima, GE Healthcare, Madison, Wisconsin) was used for continuous measurement of end-tidal carbon dioxide as well as inspired and expired isoflurane concentrations during administration.
Isoflurane dosing was followed and titrated using the alveolar concentration (%) measured in the expiratory limb of the ventilator circuit, with an exhaled isoflurane concentration of 1.6% corresponding to a minimal alveolar concentration of 1.0. Isoflurane was titrated at the discretion of the clinical team. Weaning was initiated once the patient demonstrated improvement in both ventilation and mechanical ventilation settings. After discontinuation of isoflurane, weaning of mechanical ventilation and extubation were guided by patient improvement, as determined by the multidisciplinary team.
Statistical Analysis
We used non-parametric methods to compare ventilator settings and blood gas values over time. We used univariable linear regression to describe the relationship between time on isoflurane, dose of isoflurane, age, and sex to the change in ventilator settings and blood gas values. A 2-sided P value < .05 was considered statistically significant. Statistics software (Stata 11.0, StataCorp, College Station, Texas) was used for statistical analyses.
Results
During the study period, 31 patients requiring mechanical ventilation for life-threatening bronchospasm were treated with isoflurane (Table 1). The mean duration of mechanical ventilation prior to initiation of isoflurane was 13 hours (Table 2). Mean initial isoflurane dose was an expired alveolar concentration of 0.7% (0.2–2%), and mean duration of isoflurane administration was 54.5 hours. Duration of mechanical ventilation after discontinuation of isoflurane was a mean of 180 hours (0.3–1,430 h) (see Table 2). The mean baseline pH and PCO2 were 7.13 (6.89–7.41) and 87 mm Hg (50–160), respectively (Table 3). At the time of initiation of isoflurane, mean ventilator settings included a peak inspiratory pressure (PIP) of 33 cm H2O (24–46 cm H2O), PEEP of 6 cm H2O (3–10 cm H2O), mean airway pressure of 13 cm H2O (8–24 cm H2O), and a mean FIO2 of 0.74 (0.40–1.0). Eight patients (26%) were being treated with an FIO2 of 1.0 at baseline.
Demographic Data
Ventilation and Isoflurane Treatment Data
Mean Arterial Blood Gas and Ventilator Parameters During Isoflurane Administration
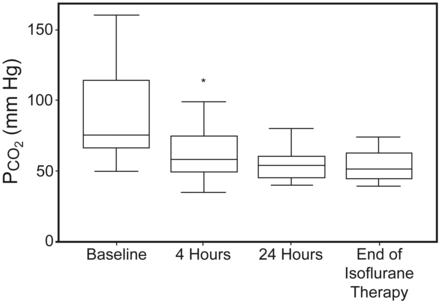
Within 4 hours of initiation of isoflurane there was significant improvement in pH and PCO2 from baseline, and significant decrease in HCO3– (P ≤ .01, Table 3, Figs. 1 and 2). Between 4 and 24 hours of treatment with isoflurane, pH continued to improve (P < .001, see Table 3), but there was no significant change in PCO2 (P = .07) or HCO3– (P = .06). For those patients treated > 24 hours, there continued to be improvement in pH and HCO3– (P < .01), but no change in PCO2 (P = .12) for the duration of therapy (see Table 3 and Figs. 1 and 2).
This figure demonstrates the pH change during isoflurane administration. This figure compares pH values at baseline (prior to isoflurane initiation) 4 hours, 24 hours, and the time of isoflurane discontinuation. The plots represent the median (line), interquartile range (box), and adjacent values (whiskers). * P = < .001 at 4 hours, compared to baseline. † P = < .001 at 24 hours, compared to 4 hours. ‡ P = < .001 at end of therapy, compared to 24 hours.
This figure demonstrates the PCO2 change during isoflurane administration. This figure compares PCO2 values at baseline (prior to isoflurane initiation), 4 hours, 24 hours, and the time of isoflurane discontinuation. The plots represent the median (line), interquartile range (box), and adjacent values (whiskers). * P = < .001 at 4 hours, compared to baseline.
Higher maximum isoflurane doses (as measured by the expired alveolar concentration) were not associated with an improvement in PCO2 or pH during isoflurane administration. There were also no differences in pH, PCO2, or any ventilator parameter during isoflurane administration related to either age or sex.
In regard to ventilator parameters, PIP did not change in the first 4 hours of therapy with isoflurane (P = .62, see Table 3). However, from 4 to 24 hours there was a statistically significant decrease in PIP, from a mean of 32 cm H2O at 4 hours to a mean of 28 cm H2O at 24 hours (P = .006, see Table 3). For patients treated with isoflurane for longer than 24 hours there was no change in PIP between 24 hours and the end of therapy (P = .73). Higher doses of isoflurane were not associated with a change in PIP (P = .23). FIO2 decreased within 4 hours of initiation of isoflurane (P < .001) and continued to improve from 4 to 24 hours (P = .02). There was no change in FIO2 from 24 hours until the end of therapy in those patients treated beyond 24 hours (P = .12, see Table 3)
Hypotension (defined as a drop in blood pressure not responsive to fluid administration and necessitating vasoactive agent administration) was the most common side effect in this series, and developed after isoflurane initiation in 24 (77%) patients. Other than the initiation of isoflurane, there were no other clinical changes or diagnoses felt to be contributing to this blood pressure instability. Most of these 24 patients were treated with dopamine monotherapy (79%), and the remainder were treated with varying combinations of dopamine, epinephrine, and phenylephrine (Table 4). Mean time to vasoactive agent initiation after starting isoflurane was 3 hours (0–16 h), and the mean maximum dose of dopamine used in these patients was 13.6 μg/kg/min (2.5–20 μg/kg/min). The mean expiratory alveolar concentration isoflurane dose upon vasoactive agent initiation was 0.6% (0.1–1.5%). There was no difference between the maximum dose of isoflurane in patients who developed hypotension and those who did not (P = .63). Fifteen of the 24 hypotensive patients (63%) had vasoactive agents discontinued prior to discontinuation of isoflurane, and there was no difference between the isoflurane dose upon initiation of vasoactive medications (0.7%), when compared to the dose when vasoactive infusions were discontinued (0.6%).
Complications During Isoflurane Administration*
Additional side effects during isoflurane administration included cardiac side effects in 3 patients. These cardiac effects encountered during isoflurane administration included one patient with supraventricular tachycardia responsive to treatment with verapamil, one patient with self-limited ST segment changes associated with a transient rhythm disturbance that was not hemodynamically important and was self-limited, and one patient with self-limited arrhythmias in the setting of having a pacemaker for underlying baseline dysrhythmias. In addition, 3 patients also developed neurologic side effects thought to be related to isoflurane, which included abnormal movements in 2 patients and withdrawal symptoms in one additional patient. All of these neurologic symptoms resolved upon discontinuation of isoflurane. Three patients had chest tubes in place prior to isoflurane initiation, and new pneumothorax was noted in one patient during administration of isoflurane. The pneumothorax developed in a patient who required treatment with PIP of > 45 cm H2O for approximately 24 hours after initiation of isoflurane. Despite these ventilatory requirements, PCO2 remained > 100 mm Hg until approximately 24 hours after initiation of mechanical ventilation and isoflurane.
Of the 6 patients treated with isoflurane for > 100 hours, 4 of them developed either arrhythmia, neurologic side effects, and/or pneumothorax. No additional side effects or complications related to isoflurane administration were noted. There were also no recorded difficulties or complications for staff members caring for patients being treated with isoflurane.
Twenty-nine of the 31 patients (94%) survived to hospital discharge. Upon discharge, 4 of the 29 survivors required ongoing treatment with oxygen, although the etiology of this persistent oxygen requirement was unclear. In the 2 non-survivors, the durations of mechanical ventilation prior to the initiation of isoflurane were 103 and 96 hours, and only one patient who survived in this series underwent mechanical ventilation prior to isoflurane initiation for longer than the 2 non-survivors (120 h).
Discussion
Patients with respiratory failure secondary to life-threatening bronchospasm who require mechanical ventilation are an extremely challenging population of PICU patients. These patients may become refractory to conventional therapies, and isoflurane is a potent bronchodilator that has been previously reported as an effective rescue therapy in adult and pediatric patients with severe bronchospasm in these most severely affected patients6,7,9–11,20
The primary limitation to the use of isoflurane in the PICU setting traditionally has been related to practical difficulties associated with administration of inhaled anesthetic agents outside of the operating room. These difficulties include problems with administration, monitoring, and gas scavenging. However, successful adaptation of standard ventilators has been reported, and recent advances in ventilator technology may facilitate the ease of administration of these agents in the PICU.10,20–22 Despite these advances, the largest reported series to date of isoflurane administration for pediatric patients with asthma is 10 children, published in 2006 by Shankar et al.20
In this series we administered isoflurane to 31 mechanically ventilated patients with clinical evidence of bronchospasm. The pH, PCO2, and FIO2 significantly improved within 4 hours of initiation of isoflurane, and this response was persistent for the duration of therapy (see Table 3 and Figs. 1 and 2). This improvement is consistent with prior reports documenting rapid improvement of blood gas parameters in bronchospastic patients treated with isoflurane.10,20,22,23 Interestingly, an initial worsening of metabolic acidosis was noted in the first 4 hours of therapy with isoflurane, demonstrating that the pH improvement during this initial time period was related to improvement of ventilation and improvement of respiratory acidosis. These improvements in pH and PCO2 were sustained over time, but the continued rise in HCO3– over time likely contributed to the substantial ongoing improvement in pH from 4 hours until the end of therapy (see Table 3 and Figs. 1 and 2).
The impact of isoflurane dose on hemodynamic status is less clear. Hypotension requiring fluid administration is common following the transition to positive-pressure ventilation, especially early in the course of illness, when both airway pressures and sedation are being titrated. However, after initial stabilization, most patients with respiratory failure do not require vasoactive medication administration. We elected to utilize initiation of vasoactive agents as the definition of hypotension in this series, in attempt to better establish the impact of isoflurane dosing on blood pressure stability.
Twenty-four of the 31 patients (77%) developed hypotension requiring vasoactive infusions during administration of isoflurane. There was no significant relationship between maximum isoflurane dose and the need for a vasoactive medication for blood pressure support. These data are supported by the fact that 15 of these 24 hypotensive patients (63%) had their vasoactive infusions discontinued while isoflurane continued, with a mean isoflurane dose of 0.6% (0.05–1.5) upon vasoactive medication discontinuation. The lack of difference between the isoflurane dose upon initiation of vasoactive infusions (0.7%) and discontinuation of these medications (0.6%) suggests that the vasodilation that occurs upon initiation of isoflurane may be short-lived, self-limited, and not sustained during prolonged administration. This early development of hypotension most likely was secondary to vasodilation upon initiation of isoflurane. In these situations, it is possible that blood pressure instability may be mitigated by prompt weaning of other sedating agents or β agonists, as allowed by the clinical situation for a given patient.
Other than hypotension, there was a relatively low incidence of additional side effects attributable to isoflurane in this series. Most of the safety data on isoflurane in children comes from its use for general anesthesia, but there have been a number of small studies demonstrating the safety of isoflurane for prolonged PICU sedation up to 7 days.24–31 In these studies, reversible neurologic dysfunction and abstinence syndrome are the most commonly reported side effects.24,25,27,32 Three patients in this series developed neurologic side effects, which are well described in the setting of prolonged isoflurane administration.24,25,33 Two patients developed abnormal movements during isoflurane administration. The maximum isoflurane doses in these patients were 0.8% and 1.8%, which does not suggest a dose-response relationship to neurologic dysfunction related to isoflurane. In addition, the duration of isoflurane administration in these 2 patients with abnormal movements were 31 and 111 hours, also not clearly demonstrating a relationship between duration of therapy and neurologic side effects. However, broad generalization is impossible, given the number of patients with neurologic side effects in this series. The third patient with neurologic side effects developed an abstinence or withdrawal syndrome including restlessness, jitteriness, and agitation upon weaning of isoflurane. The maximum dose of isoflurane in this patient was 1.1%, which was the mean maximum dose in this series, and the duration of isoflurane in this patient was 42 hours, just below the mean duration in this series. Symptoms in all 3 patients resolved as isoflurane was discontinued. Based on these limited data, it would appear that the neurologic side effects related to isoflurane administration are idiosyncratic and self-limited upon withdrawal of the agent.
Arrhythmias are another recognized complication of inhaled anesthetics, thought to be largely due to increased myocardial sensitivity to catecholamines associated with these agents.34 However, isoflurane has a much lower incidence of arrhythmias when compared to halothane.34–36 Three patients in this series developed rhythm disturbance, but one was in a patient with known arrhythmia and an implanted pacemaker. It is possible that the rhythm change in this patient was chronic in nature and unrelated to isoflurane administration. The other 2 rhythm disturbances in this series were transient, resolved with weaning of isoflurane, and did not recur.
Pneumothorax is another documented complication of mechanical ventilation, and this risk is increased substantially in the setting of bronchospasm and air trapping.37–39 In this series, only one patient developed air leak while being treated with isoflurane. Improved gas exchange and decreased levels of ventilatory support secondary to isoflurane administration are potential explanations for the low incidence of pneumothoraces in these patients. However, 3 patients had chest tubes in place upon initiation of isoflurane, and the presence of these chest tubes may have been protective from the development of further air leak. It would appear that isoflurane does not increase the rate of air leak in bronchospastic mechanically ventilated patients, but further conclusions or generalizations are unwarranted, given the numerous variables that contribute to the development of air leak in mechanically ventilated patients.
While these data are interesting and support the notion that isoflurane appears to be an effective adjunctive measure in children with life-threatening bronchospasm, this study is not without limitations. First, the retrospective nature of the data collection is not ideal. As with any retrospective report, data collection was limited by the data that were available. Management was dictated by the clinical team caring for the patient at the time, and the available data are not standardized, as they would be with a prospective protocol.
Another limitation of this study is the prolonged time period that was included in this review. While including a 15 year period allowed for the collection of the largest series of pediatric patients treated with isoflurane for life-threatening bronchospasm to date, management techniques of asthma patients evolved during this time. The general strategy for the management of these patients was based on concepts and physiologic principles that remain constant, but integration of new technology, new ventilatory strategies, and evidence may have led to variation over time. In addition, there was also variability over time in the end-tidal monitoring of the vaporizer output of isoflurane in series with the ventilator.
A crucial question and additional limitation to extrapolation and broad generalization of these data are the unclear trajectory and outcome for these patients if they had not been treated with isoflurane. There is no control group for comparison in this study, since the vast majority of patients with bronchospasm in our institution who required mechanical ventilation during this time period were treated with isoflurane. During the period of time included in this report, isoflurane was considered in all patients with clinically evident bronchospasm requiring endotracheal intubation. Institutional experience suggested that isoflurane improves bronchospasm and shortens duration of mechanical ventilation, which, coupled with the relative ease of administration of isoflurane in our PICU, most likely contributed to the relatively early initiation of this therapy when patients with severe bronchospasm required mechanical ventilation. This pattern is reflected by the mean time of mechanical ventilation prior to isoflurane administration of 13 hours, and limits the ability in this series to compare the potential toxicities associated with conventional therapy with the isoflurane patients.
However, had isoflurane not been available (as is the case in many medical centers), the outcomes of these patients are not certain. Isoflurane may have led to less total mechanical ventilation time, shorter ICU stay, and improved patient outcomes, but it is equally possible that similar outcomes could have been achieved with conventional therapeutic interventions. In addition, it is possible that the use of isoflurane may have actually prolonged the duration of mechanical ventilation. In this series, mean duration of mechanical ventilation was 10.5 days, with a median of 5.5 days, compared to a reported duration of mechanical ventilation in intubated asthmatic children of between 1.8 and 6.3 days.40–42 There were a number of patients in this series who required extremely long durations of mechanical ventilation, and the majority of this time included treatment with isoflurane and post-isoflurane mechanical ventilation. It is likely that disease severity contributed to this prolonged duration of mechanical ventilation in these patients, but the impact of isoflurane on the overall management of these patients remains uncertain. In addition, the high incidence of hypotension requiring intervention is an important additional consideration as this therapy is considered.
These data support the notion that isoflurane provides bronchodilator effects for mechanically ventilated patients and may be a beneficial intervention in patients with life-threatening bronchospasm refractory to conventional therapies. Further prospective assessment of this therapeutic option would be difficult, given institutional variation and limited numbers of patients, but systematic investigation could help clarify some of the unanswered questions surrounding the use of isoflurane in this setting.
Conclusions
Isoflurane can be administered in the PICU for mechanically ventilated pediatric patients with life-threatening bronchospasm, and appears to be efficacious in relieving bronchospasm and improving ventilation, but is associated with a high potential in this context for transient hypotension and arrhythmias. These complications are often self-limited or manageable by pharmacologic means without discontinuation of isoflurane. The clinical impact of isoflurane in the context of life-threatening bronchospasm requiring mechanical ventilation remains unclear, and vigilance is needed during its administration. As technological advances improve the potential ability of centers to provide this therapy in the PICU, further systematic evaluation of this therapy is warranted.
Footnotes
- Correspondence: David A Turner MD, Division of Pediatric Critical Care Medicine, Department of Pediatrics, Duke Children's Hospital, Duke University Medical Center, Box 3046, Durham NC 27710. E-mail: david.turner{at}duke.edu.
Dr Smith was partly supported by National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development grants 1K23HD060040–01 and 1R18AE000028–01.
The authors have disclosed no conflicts of interest.
See the Related Editorial on Page 1982
- Copyright © 2012 by Daedalus Enterprises Inc.