Abstract
BACKGROUND: A correlation has been observed between obstructive sleep events and sleep quality. The aim of the study was to assess if there is also a correlation between nocturnal hypoxemia and hypercapnia and sleep efficiency and sleep fragmentation in children.
METHODS: Nocturnal pulse oximetry (SpO2) and transcutaneous carbon dioxide (PtcCO2) recordings with simultaneous actigraphy were performed in 38 children with nocturnal hypoxemia and hypercapnia during spontaneous breathing (nocturnal hypoventilation [NH] group), 25 children with partially corrected nocturnal hypoventilation (PC-NH group), and 11 subjects with normal nocturnal gas exchange (no-NH group).
RESULTS: Sleep efficiency and sleep fragmentation on actigraphy correlated with minimal SpO2 (r2 = 0.21, P = .004, and r2 = −0.10, P = .050, respectively) and the percentage of night time with SpO2 < 90% (r2 = −0.33, P < .001, and r2 = 0.13, P = .028, respectively) in the NH group. Sleep efficiency and sleep fragmentation also correlated with pulse rate standard deviation (r2 = −0.42, P < .001, and r2 = 0.37, P < .001, respectively). No correlation was observed between sleep efficiency and sleep fragmentation and PtcCO2. No correlation was observed between sleep efficiency and sleep fragmentation and SpO2, PtcCO2, and pulse rate in the PC-NH group. Sleep efficiency, sleep fragmentation, and nocturnal SpO2, and PtcCO2 were all normal and not correlated in the no-NH group.
CONCLUSIONS: In children with nocturnal hypoventilation, nocturnal hypoxemia but not hypercapnia correlates with sleep efficiency and sleep fragmentation on actigraphy.
Introduction
Obstructive sleep apnea (OSA) is a common disorder in children, characterized by repetitive episodes of upper airway narrowing and breathing pauses.1 Its main cause is hypertrophy of the adenoids and the tonsils. Obstructive apneas are often followed by an arousal, leading to sleep fragmentation and poor sleep quality. During an apnea, slowing of the heart rate may occur, with an acceleration of heart rate during the arousal, due to the activation of the sympathetic nervous system.2,3 The most severe OSA events may be accompanied by nocturnal desaturations and periods of alveolar hypoventilation with hypercapnia.
Children with neuromuscular or lung disease may present with periods of alveolar hypoventilation during sleep in the absence of obstructive events. The demonstration of nocturnal hypoxia and hypercapnia in these patients constitutes an indication for noninvasive ventilation (NIV), which aims to normalize alveolar ventilation.4–7 However, sleep quality has been poorly studied in these patients, and, in particular, the correlation between nocturnal gas exchange and sleep efficiency during spontaneous breathing and NIV.
Polysomnography (PSG) is widely accepted as the gold standard for the diagnosis of OSA, and the apnea-hypopnea index is generally used for the quantification of OSA severity.1 As such, the deleterious consequences of OSA have been mainly correlated to this index, and to a lesser extent to gas exchange anomalies. PSG is time consuming, expensive, technically demanding, and not feasible on a routine basis. Simpler alternative investigations are thus necessary for the recording of sleep characteristics of larger populations. One of these alternatives is actigraphy, which consists of wrist movement recording by a piezoelectric accelerometer. Actigraphy has been shown to give an acceptable estimation for sleep efficiency and number of awakenings in adults8,9 and children.10,11 Even if it is admitted that actigraphy provides only a fair indication of the level of arousal from sleep in children, by identifying mainly arousals accompanied by movements, and that the fragmentation index from this technique is not as accurate as the traditional sleep fragmentation measured on PSG, the simplicity of its use may be helpful to evaluate sleep quality outside the sleep laboratory.11–13 The diagnosis of alveolar hypoventilation requires the recording of pulse oximetry (SpO2) or transcutaneous oxygen (PtcO2) and transcutaneous carbon dioxide (PtcCO2) or exhaled CO2. New simple devices are available that allow a continuous noninvasive monitoring of CO2. One of these devices is the SenTec Digital Monitor (SenTec, Therwil, Switzerland), which measures SpO2 and PtcCO2 and has been validated in children.14 As pulse rate variability is a well-known index of sleep fragmentation, this variable may be analyzed simultaneously to complete the actigraphy data.15
The aim of our study was to analyze the correlation between objective sleep quality evaluated on actigraphy, nocturnal hypoxemia and hypercapnia, and pulse rate variability in children having various chronic conditions, by means of 2 simple noninvasive devices, a SpO2/PtcCO2 monitor and actigraphy. Indeed, our hypothesis is that actigraphic sleep efficiency and sleep fragmentation correlate with nocturnal hypoxemia and hypercapnia, independently of the underlying disease. As such, the nocturnal gas exchange and actigraphic data of children with overt nocturnal hypoventilation were compared to those of children with partially corrected and normal nocturnal gas exchange.
QUICK LOOK
Current knowledge
There is a correlation between obstructive sleep events and sleep quality in both adults and children. The correlation between nocturnal hypoxemia and hypercapnia and sleep efficiency and sleep fragmentation in children is not well described.
What this paper contributes to our knowledge
In children with nocturnal hypoventilation, nocturnal hypoxemia correlates with sleep efficiency and sleep fragmentation on actigraphy. A similar correlation was not seen for hypercapnia.
Methods
Subjects
The study was performed in a university pediatric pulmonology department, in children who were candidates for NIV because of various underlying conditions responsible for abnormal nocturnal gas exchange. As the aim of our study was to analyze the correlation between nocturnal hypoxemia and hypercapnia and objective sleep quality, consecutive patients were recruited prospectively and classified a priori into 3 groups, as follows. Children on any medication that could affect sleep were excluded. The first group comprised 38 consecutive children with nocturnal alveolar hypoventilation, defined by nocturnal hypoxemia and hypercapnia (NH group). Nocturnal hypoventilation was defined by a minimal SpO2 < 90% for at least 5 consecutive minutes and/or for > 10% of night time, and a PtcCO2 > 50 mm Hg for at least 5 consecutive minutes and/or > 10% of night time. Criteria for NIV were nocturnal hypoxemia with an SpO2 ≤ 90% for ≥ 10% of nocturnal recording time and/or nocturnal hypercapnia with a PtcCO2 ≥ 50 mm Hg for ≥ 10% of night time. The second group comprised 25 other subjects with partially corrected nocturnal hypoventilation (PC-NH group): that is, subjects evaluated during the first days of adaptation to NIV, when an incomplete correction of nocturnal hypoxemia and hypercapnia was observed. The last group of subjects comprised patients without nocturnal hypoventilation, defined as nocturnal SpO2 > 90% and PtcCO2 < 50 mm Hg. This group comprised 11 subjects, 7 subjects perfectly well controlled by NIV, and 4 subjects with cured nocturnal alveolar hypoventilation (no-NH group). The subjects suffered from various diseases, listed in Table 1. In order to have a homogenous population with regard to daytime gas exchange, only subjects with normal daytime blood gases were included.16 Although the sleep recordings could have been performed at home,17 all the recordings were performed in a single quiet room of the pediatric pulmonology department.
Recordings and Characteristics of the Subjects
Exclusion criteria were: age < 1 year, because the ear clip of the combined SpO2/PtcCO2 monitor is too large for small infants; dark skin, because of inaccurate SpO2 values, as has been observed with other devices18; and impaired mobility of the upper limbs, which may underestimate actigraphy data.
All the parents and, if possible, the subjects gave informed written consent for the study, which was approved by the local ethical committee.
Overnight SpO2 and PtcCO2 Recording by the Combined SpO2/PtcCO2 Monitor
The overnight SpO2 and PtcCO2 recording was performed by a monitor (SenTec Digital Monitor, software version SMB SW-V06.10, MPB SW-V04.03, V-Sign sensor and ear clip, SenTec, Therwil, Switzerland). This fully digital sensor combines the elements of an electrochemical Severinghaus-type CO2 tension sensor with the optical elements of a conventional SpO2 sensor, thus providing noninvasive and continuous PtcCO2 and SpO2 monitoring. Prior to the application of the sensor to the subject, the sensor was prepared and calibrated as per the manufacturer's recommendations. The sensor was then applied to the subject's earlobe. Effective recording was started after 15 min, which is the time required for the stabilization of the PtcCO2 and SpO2 values.14 All the recordings were performed in room air.
Overnight Actigraphy
During the same night, actigraphy recording (Actiwatch, Cambridge Neurotechnology, Cambridge, United Kingdom) was performed at the non-dominant wrist. Actigraphy settings were chosen so that data were stored in 1-minute epochs. Actiwatch is a lightweight, wrist-worn activity monitoring system in which an accelerometer monitors the occurrence and extent of motion. The extent and speed of motion are integrated to produce activity counts or the number of times per epoch that the signal crosses threshold (set very close to zero). Activity count is a generic term used to denote the amplitude of the signal produced by the accelerometer in the Actiwatch. The number of counts is proportional to the intensity of the movement. The system works as follows: a bar shaped accelerometer flexes when the Actiwatch is moved, producing a voltage in the sensor. The degree and force of flexing influences the voltage, which is translated into activity counts.
Data Analysis
The combined SpO2/PtcCO2 monitor and actigraphy recordings were started simultaneously, at usual bed time, and care was taken to respect the subject's sleep. The monitoring lasted at least 6 hours, and sleep and the correct positioning of the ear clip were regularly checked by the attending nurse during the night.
The combined SpO2/PtcCO2 monitor used a fixed 3-second averaging time for pulse rate and stored in memory a new value every 2 seconds. SpO2, pulse rate, and PtcCO2 were extracted and analyzed using software (version SMB SW-V06.10, MPB SW-V04.03). This program provided metrics for oxygenation, pulse rate, pulse rate variability, and PtcCO2, and calculated: mean SpO2; minimum SpO2; number of desaturations ≥ 4%/h of study; percent of time spent with SpO2 < 92% and 90%; mean and maximum PtcCO2; the time spent with PtcCO2 > 50 mm Hg; mean, median, minimum, maximum, and standard deviation of pulse rate; and the frequency of pulse rate rises by > 6, 10, and 15 beats/min (pulse rate index [PRRI-6, PRRI-10, PRRI-15]). The standard deviation (SD) of pulse rate was used to estimate pulse rate variability above the entire recording. Periods of SpO2/PtcCO2 and actigraphy recording were excluded from analysis if the SpO2 quality signal indicated a low signal, low perfusion, unrecognized, defective, or no sensor, or interference.
The actigraphy data were assessed using the algorithm supplied by the Actiwatch sleep analysis software. The Actiwatch arousal threshold was set at an integrated activity count of 40 movements within a 1-minute epoch. Light off and light on were determined either using the information from the Actiwatch event marker or by the diary of the attending nurse. Sleep onset was determined manually by the scorer as initiation of a sequence of 10 or more consecutive epochs of inactivity after light off. End of sleep was defined as the start of a sequence of 10 consecutive minutes containing activity counts above the threshold of 40 activity counts at get-up time, followed by continuous activity. Sleep efficiency was calculated by the program as the percentage of time spent asleep between sleep onset and sleep offset. Fragmentation index measures restlessness, and was calculated by summing the percentage of minutes spent moving with the percentage time spent in the immobility phase per minute.
The computer of the SenTec monitor and the computer setting up the actigraph were synchronized to a standard time.
Statistical Analysis
Data are presented as mean ± SD. Comparisons between groups were conducted using one way analysis of variance, followed by a pair-wise multiple comparison test (Holm-Sidak test). Correlations between the gas exchange data (SpO2 and PtcCO2), the actigraphy data (sleep efficiency and fragmentation index), and pulse rate were assessed using simple linear regression analysis. A P value of < .05 was considered statistically significant.
Results
Subjects
The underlying conditions of the subjects are summarized in Table 1. The mean age of the subjects was comparable among the 3 groups. The majority of the subjects suffered from upper-airway obstruction, lung disease, or thoracic deformity.
Overnight SpO2, PtcCO2, and Actigraphy Data
Mean actual sleep time, sleep efficiency and fragmentation index were significantly better in the no-NH group, as compared to the NH group, the PC-NH group harboring intermediate results (Table 2). Table 3 shows the gas exchange and pulse rate data of the 3 groups of subjects. As expected, minimal SpO2 and the percent of time spent with a PtcCO2 > 50 mm Hg were significantly better in the no-NH group, as compared to the NH group, with the PC-NH group harboring again intermediate results. There was a trend for a lower percentage of time spent with SpO2 < 90% and 92%, a lower desaturation index, and a lower maximal PtcCO2 in the no-NH group, as compared to the 2 other groups, but the difference was not statistically different. The pulse rate data did not differ among the 3 groups, except for PRRI-6, which was significantly higher in the no-NH group, as compared to the 2 other groups.
Actigraphy Data
Gas Exchange and Pulse Rate Data
Correlation Between Overnight SpO2, PtcCO2, and Actigraphy Data
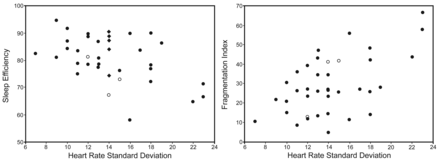
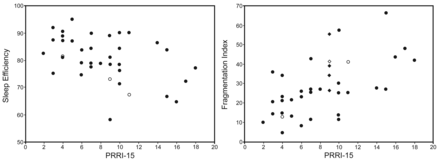
In the NH group, sleep efficiency and fragmentation index correlated with minimal SpO2 (r2 = 0.21, P = .004, and r2 = −0.10, P = .050, respectively) (Fig. 1) and the night time spent with SpO2 < 90% (r2 = −0.33, P < .001, and r2 = 0.13, P = .028, respectively). Sleep efficiency and sleep fragmentation correlated also with standard deviation of pulse rate (r2 = −0.42, P < .001, and r2 = 0.37, P < .001, respectively) (Fig. 2), and the frequency of PRRI-6 (r2 = −0.33, P < .001, and r2 = 0.13, P = .028, respectively), PRRI-10 (r2 = −0.33, P < .001, and r2 = 0.15, P = .034, respectively), and PRRI-15 (r2 = −0.33, P < .001, and r2 = 0.33, P = .007, respectively) (Fig. 3). No correlation was observed between sleep efficiency and sleep fragmentation and PtcCO2.
Left: Correlation between minimum SpO2 and sleep efficiency, assessed by wrist actigraphy in children with nocturnal hypoventilation. Right: Correlation between minimum SpO2 and fragmentation index, assessed by wrist actigraphy in children with nocturnal hypoventilation. The 3 subjects with the most severe nocturnal hypoxemia are represented by open circles.
Left: Correlation between pulse rate standard deviation and sleep efficiency, assessed by wrist actigraphy in children with nocturnal hypoventilation. Right: Correlation between pulse rate standard deviation and fragmentation index assessed by wrist actigraphy in children with nocturnal hypoventilation. The 3 subjects with the most severe nocturnal hypoxemia are represented by open circles.
Left: Correlation between the frequency of pulse rate rises by more than 15 beats/min and sleep efficiency, assessed by wrist actigraphy in children with nocturnal hypoventilation. Right: Correlation between the frequency of pulse rate rises by > 15 beats/min and fragmentation index assessed by wrist actigraphy in children with nocturnal hypoventilation. The 3 subjects with the most severe nocturnal hypoxemia are represented by open circles.
In the PC-NH group, despite the presence of mild nocturnal hypoxemia and hypercapnia, no correlation was observed between sleep efficiency and sleep fragmentation and SpO2, PtcCO2, and pulse rate variability.
In the no-NH group, sleep efficiency, sleep fragmentation, SpO2, and PtcCO2 were all within the normal range and were not correlated.
Discussion
This study is the first to analyze the correlation between nocturnal gas exchange and objective sleep quality determined on actigraphy in children having different levels of alveolar hypoventilation. The main observations of our study are that: in children with nocturnal hypoventilation, nocturnal hypoxemia, but not hypercapnia, is associated with lower sleep efficiency and a higher fragmentation index; and that nocturnal gas exchange, sleep efficiency, and sleep fragmentation may improve during NIV or after the cure of the underlying disease.
Our hypothesis was that nocturnal hypoxemia and hypercapnia, independently of the underlying disorder, are associated with lower sleep efficiency and greater sleep fragmentation. However, we observed this correlation only for nocturnal hypoxemia and not for nocturnal hypercapnia. This may be explained by the rapidity of SpO2 changes, as compared to the more progressive and less variable PtcCO2 changes.19 Another hypothesis may be that nocturnal hypercapnia does not impair sleep efficiency in children to the same extent as SpO2. Indeed, whatever the analyses of PtcCO2 (mean value, maximal value, or amount of time spent above 50 mm Hg), we were not able to demonstrate any effect of PtcCO2 on actigraphy sleep efficiency or sleep fragmentation.
The correlation of nocturnal hypoxemia and sleep efficiency and sleep fragmentation was observed only in the NH group, who had the most severe alterations in nocturnal gas exchange. The PC-NH group had mild alveolar hypoventilation because the subjects were not adapted to NIV. The lack of correlation of nocturnal hypoxemia and sleep efficiency and sleep fragmentation in this group may be explained by the mild impairment in gas exchange, the small number of subjects, or the presence of the ventilator. Indeed, the presence of the ventilator acts as an external event interfering with the subject's gas exchange and sleep quality.19 During NIV, patient-ventilator synchrony or leaks may affect sleep quality, as a correlation has been observed between the severity of nocturnal desaturation and patient-ventilator asynchrony.20 The negative impact of unintentional leaks during long-term NIV on sleep architecture, as well as the improvement in sleep quality after the correction of leaks, has been shown in several studies.21,22 However, subjective sleep quality may be normal in children during long-term NIV, even if they remain hypercapnic,14 and the objective sleep quality was normal in the 7 subjects who did not have any residual nocturnal hypoventilation with NIV in the present study.
Most studies analyzing the deleterious consequences of sleep-disordered breathing have concerned children with OSA. OSA is associated with poor sleep quality and cardiovascular stress. Indeed, pulse rate variability is higher in children with OSA, as compared to controls,23,24 and decreases after adenotonsillectomy.25 This seems to be caused by an increased sympathetic activity, as documented by the attenuation of peripheral arterial tonometry, a noninvasive measure of moment to moment sympathetic nerve activity, in children with OSA.26,27 In the present study, the cardiovascular consequences of poor sleep quality and sleep fragmentation were analyzed by means of pulse rate variability and PRRI. Normal values for pulse rate variability in children have been published.28 In healthy subjects, pulse rate variability is related to respiratory sinusal arrhythmia due to parasympathetic modulation. Conversely, pulse rate variability is due to sympathetic activation in patients with upper-airway obstruction. In the present study, we analyzed subjects with moderate nocturnal hypoxemia and hypercapnia during SB. Most of these subjects had upper-airway obstruction or lung disorders characterized by an increase in respiratory load.29,30 This increase in respiratory load is associated with an increase in intrathoracic pressure changes and sympathetic activation. The correlation of sleep efficiency and sleep fragmentation with pulse rate variability in these subjects may thus be explained by the presence of upper-airway obstruction and/or increase in respiratory load. Also, the intermediate sleep efficiency and sleep fragmentation values in the PC-NH group may be explained by the incomplete correction of the respiratory load.
It is probable that the comparable PRRI-10 and PRRI-15 values in the 3 groups of subjects underlie a different mechanism. As such, an analysis in domain frequency would be of interest to differentiate the mechanisms of pulse rate variability in these groups of subjects. Indeed, a noninvasive analysis of heart rate variability based on noninvasive electrocardiogram recording would be very contributive within this context.31 But, most importantly, a correlation between pulse rate variability and sleep quality was observed only in the NH group.
Sleep quality was evaluated by actigraphy and not by PSG. PSG is the gold standard for the diagnosis of sleep-disordered breathing, but this investigation is technically demanding and difficult to perform on a routine basis in a pediatric pulmonology department. For this reason, we took the option to combine nocturnal actigraphy with simultaneous recording of gas exchange. Because of its minimal invasiveness and excellent local tolerance, this monitoring may better respect natural sleep in children.17 Previous studies have reported a close agreement between PSG and actigraphy for total sleep time and sleep efficiency in children and adults.9,10 However, actigraphy provides a less reliable indication of the level of arousal from sleep in children than PSG11 and may also overestimate sleep latency, total sleep time, and sleep efficiency.12 But the aim of our study was not to diagnose OSA, but to look for a correlation between nocturnal gas exchanges and simple and easy indices of sleep efficiency and sleep fragmentation. Of note, none of the subjects had severe nocturnal hypoxemia or hypercapnia (see Table 3).
Sleeping in an unfamiliar environment such as a hospital may affect sleep quality. Although SpO2/PtcCO2 recordings and actigraphy may be performed at home,17 we took the option to perform all the recordings in the hospital. An acclimatization effect on sleep quality was improbable because all the subjects had slept in the pediatric department al least once before the study. None of the subjects was hypoxemic or hypercapnic while awake, which excludes an effect of chronic daytime hypercapnia or hypoxemia on sleep quality. However, the maximal changes in SpO2 and PtcCO2 between awake and sleep may have differed between subjects and may affect sleep quality, as well as the cause of nocturnal hypoxemia and/or hypercapnia. The heterogeneity of the population may be considered as a limitation of the study, as other factors, such as nocturnal cough for a patient with cystic fibrosis, or position for a patient with OSA syndrome, may affect sleep quality. But this heterogeneity may also strengthen our results, as the correlation between nocturnal hypoxemia and sleep quality was observed for the population as a whole. NIV may affect sleep quality, as discussed above, but it may also improve sleep quality, either directly, by suppressing the events causing an arousal, or artificially, by restricting the patient's movements. However, the sleep parameters of the 7 subjects perfectly well controlled by NIV were not significantly different from the 5 subjects with normal nocturnal gas exchange after the cure of the primary disease. These results also show that nocturnal gas exchange and sleep quality may be normal during NIV in children, allowing us, in our opinion, to combine the results of these 2 groups of subjects. We identified the 3 subjects with the most severe nocturnal hypoxemia with a different symbol (see Figs. 1 to 3). Indeed, the correlation of minimal SpO2 with sleep efficiency and the fragmentation index was no longer significant after the exclusion of these patients, underlining the necessity of the confirmation of our data in a larger group of subjects with severe nocturnal hypoxemia. Finally, nocturnal CO2 was analyzed by means of a transcutaneous monitor, which has been validated in our laboratory but whose time response is slower, compared to capnography.
Conclusions
In conclusion, this study is the first to show by means of 2 simple noninvasive devices monitoring gas exchange and wrist movements, that nocturnal hypoxemia, but not nocturnal hypercapnia, correlates with sleep efficiency and sleep fragmentation and pulse rate variability in children with nocturnal hypoventilation. However, a validation of this observation by standard PSG in larger groups of children, before and after treatments such as NIV, is warranted.
Footnotes
- Correspondence: Brigitte Fauroux MD PhD, Assistance Publique - Hôpitaux de Paris, Hôpital Armand Trousseau, Pediatric Pulmonary Department, Université Pierre et Marie Curie - Paris 6, INSERM U955, 28 Avenue du Docteur Arnold Netter, Paris, France F-75012 France. E-mail: brigitte.fauroux{at}trs.aphp.fr.
Dr Fauroux was partly supported by the Association Française Contre les Myopathies, ADEP Assistance, Suresnes, France. Dr Krivec was partly supported by the European Respiratory Society, and grant 70153 from the University Medical Centre, Ljubljana, Slovenia.
The authors have disclosed no conflicts of interest.
- Copyright © 2012 by Daedalus Enterprises Inc.