Introduction
Patients with septic shock and respiratory failure often require hemodynamic monitoring in order to guide bedside management. This is particularly true of patients with dialysis-dependent chronic kidney disease, who may be less tolerant of large volume resuscitation than individuals with normal renal function. With the declining use of pulmonary artery catheters and the introduction of early goal-directed therapy as a means for resuscitating patients with septic shock,1 central venous catheters (CVCs) are increasingly being used for this purpose. These catheters allow continuous monitoring of central venous pressure to guide fluid administration, as well as point measurement or continuous monitoring of central venous oxygen saturation (ScvO2), a surrogate measure of mixed venous oxygen saturation (SvO2) that provides information about the balance between oxygen delivery and consumption at the tissue level. In order to use the central venous pressure and ScvO2 to guide sepsis resuscitation or other aspects of patient management, the clinician must verify that the line is in the appropriate position and ensure there are no sources of error in the generated data. We present a case of a patient with chronic kidney disease who presented with sepsis and respiratory failure, which demonstrates some of the challenges that can arise in these regards and how to approach these situations.
Case Summary
A 54-year-old man with a past medical history notable for dialysis-dependent chronic kidney disease, diabetes mellitus, and hypertension presented to the emergency department with shortness of breath and hypotension. Soon after arrival he became drowsy and was placed on noninvasive mechanical ventilation. Labs drawn in the emergency department revealed white-blood-cell count 22,000 cells/μL, with 90% neutrophils and 1% bands, blood urea nitrogen 67 mg/dL, creatinine 8.1 mg/dL, hematocrit 34%, and venous lactate 1.2 mmol/L. His Acute Physiology and Chronic Health Evaluation II score at this time, calculated retrospectively, was 29. Following admission to the ICU, a venous blood gas analysis revealed pH 7.10, PCO2 82 mm Hg, and PO2 52 mm Hg. The patient was subsequently intubated with a 7.5-mm endotracheal tube, using a rapid sequence protocol with etomidate for sedation and rocuronium for neuromuscular blockade. He was placed on mechanical ventilation with the following settings: volume assist control, respiratory rate 24 breaths/min, tidal volume 510 mL (8 mL/kg ideal body weight), FIO2 1.0, and PEEP 5 cm H2O. Peak inspiratory pressure at the time was 53 cm H2O, while mean airway pressure was 13 cm H2O. Sedation and analgesia for ventilator tolerance were provided with intravenous boluses of fentanyl and midazolam, administered according to a nurse-driven protocol. His vital signs following intubation were: temperature 36.7°C, heart rate 111 beats/min, blood pressure 87/39 mm Hg, and SpO2 100%. On examination, the patient had breath sounds bilaterally, without crackles or wheezes. He demonstrated an irregular tachycardic rhythm, with no murmurs, rubs, S3, S4, or loud P2. His abdomen and skin were unremarkable, and bilateral lower extremities showed symmetric 1+ pitting edema. The patient had bilateral upper extremity dialysis fistulae, both of which demonstrated a palpable thrill. Patient records available at this time indicated that the left upper extremity fistula had a pseudoaneurysm.
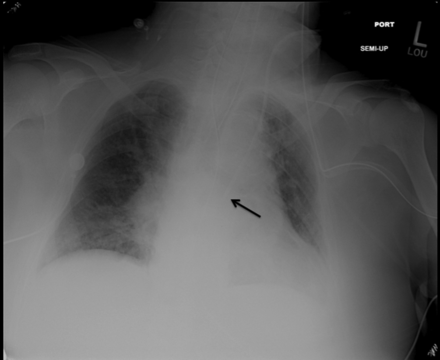
A triple lumen CVC capable of continuously monitoring ScvO2 (Presep, Edwards Lifescience, Irvine, California) was placed in the left internal jugular vein without difficulty, using ultrasound guidance. The left internal jugular was chosen because the physician performing the procedure, aware of the pseudoaneurysm of the left-sided fistula, assumed the patient received hemodialysis via the right upper extremity dialysis fistula and wanted to avoid cannulation on that side. After the portable chest radiograph obtained to confirm catheter tip position (Figure) raised concern about possible intra-arterial placement, a pressure transducer was attached to the catheter (Ultraview, Spacelabs Healthcare, Issaquah, Washington) and revealed a central venous pressure waveform with a pressure of 17 mm Hg. The CVC was then connected to the continuous ScvO2 monitoring system (Vigilance II, Edwards Lifescience, Irvine, California), and the initial reading was 94%. A blood gas analysis sample was obtained from the central line to calibrate the device and revealed pH 7.39, PCO2 33 mm Hg, PO2 96 mm Hg, and oxygen saturation 96%.
Portable chest radiograph taken after insertion of the central venous catheter in the left in internal jugular vein. The black arrow points to the tip of the catheter.
Based on the results of pressure transduction and chest radiography, it was felt that the catheter tip was likely in a persistent left superior vena cava, proximal to the right atrium. The ScvO2 was felt to be elevated due to the presence of a dialysis fistula on the same side as the catheter, through which arterialized blood entered the venous system and traveled past the catheter tip. ScvO2 monitoring was discontinued, and the central line was used only for infusion of vasopressors and other medications.
The patient required a continuous infusion of norepinephrine to achieve a goal mean arterial pressure of > 65 mm Hg. The infusion rate varied between 6 and 10 μg/min before it was discontinued on hospital day 2, as his hemodynamic compromise resolved (the day of presentation is treated as day zero). Following admission, the patient had been started on vancomycin, ceftriaxone, and azithromycin pending culture results. Blood cultures drawn in the emergency department subsequently grew group B β-hemolytic streptococci on hospital day 2, and his antibiotic coverage was narrowed to penicillin on hospital day 3. Beginning on hospital day 1, the patient underwent intermittent hemodialysis, with minimal hemodynamic instability on the norepinephrine infusion. He was dialyzed using his left upper extremity fistula after the nephrologist ascertained that this was his usable fistula.
With intermittent hemodialysis and treatment of his infection, the patient's oxygenation improved, such that his FIO2 was 0.4 on hospital day 1, and 0.3 on hospital day 2. The patient received spontaneous breathing trials (CPAP 5 cm H2O with no pressure support) starting on hospital day 1, but failed on this and the following day due to a rapid shallow breathing pattern. On hospital day 3 he maintained a respiratory rate of 25 breaths/min and a tidal volume of 330 mL during his spontaneous breathing trial, and was subsequently extubated to oxygen by nasal cannula. The central line was removed following extubation, and the patient was transferred to the medicine ward on hospital day 4. He was subsequently discharged from the hospital on hospital day 5 with plans to complete a 14-day course of intravenous cefazolin, dosed with hemodialysis.
Discussion
CVCs play a key role in the management of patients with septic shock and respiratory failure. Our case demonstrates some of the challenges that can arise with placement of these devices and interpretation of some of the data provided by them.
The first challenge associated with the CVC is safe and correct placement of the device. The most important step in this regard is to ensure that the introducer needle and guide wire are in the central vein, rather than an artery, prior to insertion of the CVC. This is done using portable ultrasound to identify the vein prior to needle puncture, to observe the needle entering the appropriate vessel, and to confirm venous placement of the guide wire prior to inserting the catheter. Before advancing the guide wire through the needle, clinicians can also use pressure transduction via the 18-gauge needle to verify the presence of a central venous pressure waveform. In our case we used ultrasound guidance, as described above, but did not use pressure transduction prior to guide wire insertion. Catheter placement proceeded without apparent complication.
Once the CVC has been secured in place, chest radiography is performed to confirm that the tip of the CVC is in the appropriate position at the junction of the superior vena cava and the right atrium. In our patient the chest radiograph and the initial, uncalibrated ScvO2 reading were both concerning for a possible intra-arterial placement. In such situations, providers can employ several tactics to verify that the CVC is in a central vein. Pressure transduction can be used at this point to identify either an arterial or venous waveform, although, as noted above, this measurement is more appropriately performed prior to insertion of the guide wire and CVC. Second, comparisons can be made between simultaneous blood gases drawn from the CVC and a known arterial site. Similar values on the 2 samples are indicative of intra-arterial placement, while substantial differences, particularly with regard to the PO2, are supportive of intravenous placement. Finally, bedside ultrasound and color Doppler can be used to visualize the CVC in a non-pulsatile vessel. In our patient, pressure transduction and bedside ultrasound were both consistent with central venous placement.
After reviewing the chest radiograph and the blood gas data, we suspected that our patient had a persistent left superior vena cava, a rare anatomical variation, occurring in only 0.3–0.5% of the population.2 This could have been confirmed using a chest computed tomography scan, but we opted not do to this, as the scan results would not have changed patient management. A persistent left superior vena cava empties into an enlarged coronary sinus and is usually accompanied by a right superior vena cava. The vessel can also drain into the left atrium and lead to right-to-left shunt and hypoxemia.3 It is most frequently discovered after accessing a central vein, either for line placement or during electrophysiology procedures.4,5 Patients with this anomaly may also have a higher incidence of conduction system abnormalities and arrhythmias,6 although none of these issues were seen with our patient.
The elevated oxygen saturation seen on the ScvO2 monitor and on the blood gas drawn from the CVC were likely due to the ipsilateral dialysis fistula and the fact that arterialized blood was being returned to the venous circulation upstream of the catheter tip, thereby raising the saturation and PO2 from true venous levels. Another possible anatomic variant that could explain the elevated oxygen saturation seen on the sample drawn from the CVC is that of partial anomalous pulmonary venous connection. This entity includes several usually asymptomatic anatomic variants that cause oxygenated blood from the pulmonary venous system to drain into the systemic venous system. Common variations include the left upper pulmonary vein draining into the left innominate vein, drainage of smaller pulmonary vessels from the right upper lobe into the superior vena cava, and pulmonary venous drainage into the azygous vein, coronary sinus, and inferior vena cava. In the case of the scimitar syndrome, the entire right lung (or sometimes a portion of it) drains into pulmonary veins and then the scimitar vein, which subsequently drains into the inferior vena cava. Of note, the presence of both persistent left superior vena cava and anomalous pulmonary venous return in the same individual has been reported frequently,7 and this finding can be asymptomatic.8 Confirmation of partial anomalous pulmonary venous connection is generally made with advanced imaging, such as echocardiography, computed tomography, or magnetic resonance imaging. Cardiac catheterization with oximetry can also be performed to evaluate the degree of shunt volume. We did not consider this diagnosis at the time of the patient's admission, and, as a result, did not pursue studies to confirm or rule out this possibility.
Once the CVC is safely in place and catheter tip position has been confirmed, the clinician can monitor ScvO2 as a means to guide resuscitation of the patient. The ScvO2 can be obtained as a point measurement off a blood gas drawn from the CVC or monitored continuously using a proprietary catheter and monitor (Presep or Vigilance II, Edwards Lifesciences, Irvine, California), The ScvO2 serves as a surrogate measure of the Sv̄O2, which provides information about the balance between oxygen delivery and oxygen consumption in the tissues.9 Because measurement of the Sv̄O2 requires a pulmonary artery catheter, and use of such devices has fallen off in the past decade, following studies questioning their impact on patient outcomes, ScvO2 has become a more commonly followed resuscitation end point.
Although monitoring of ScvO2 is easy to do and provides ready feedback regarding the impact of various interventions, there are important limitations of the parameter about which the user must be aware. First, as our case demonstrates, clinicians should be wary of using the ScvO2 to guide resuscitation in patients with dialysis fistulas or partial anomalous pulmonary venous connection. In such cases the reported values will be falsely elevated and, as a result, will not accurately reflect the balance between oxygen delivery and consumption in the tissues. In the case of partial anomalous pulmonary venous connection, the magnitude of the error will depend on the shunt volume associated with the particular anomaly. For a dialysis fistula the magnitude of the error will be affected by the blood flow through the fistula and the downstream position of the catheter tip relative to the fistula and right atrium. The greater the flow through the fistula, the more likely that a high oxygen saturation will be measured from the catheter tip. Blood flow through a dialysis fistula has been reported to average between 1.3 and 2.7 L/min, depending on whether the patient has a brachial or radial fistula and whether or not they have pulmonary hypertension, and may even range as high as 3.3 L/min.10
In terms of positioning, the CVC must be downstream of the fistula for the ScvO2 to be affected. This is illustrated in a report of a patient with a right-sided hemodialysis fistula, who had 2 catheters placed in his right internal jugular vein. The first was a short introducer catheter whose tip remained in the internal jugular vein and, as a result, was not in contact with arterialized blood coming from the dialysis fistula via the subclavian vein. The second was a CVC whose tip was more centrally located in the superior vena cava and directly in the path of returning arterialized blood from the subclavian vein. The oxygen saturation measured by CO-oximetry off the catheter residing in the internal jugular vein was 70.9%, while the corresponding value measured from the catheter in the superior vena cava was 96.7%.11
The degree of elevation in the ScvO2 will also depend on the positioning of the catheter tip relative to the fistula and the right atrium; the closer the tip is to the dialysis fistula, the greater the amount of arterialized blood flowing by the catheter tip, and, as a result, the higher the measured oxygen saturation. If the catheter tip resides in the right atrium, the effect of the fistula will be reduced, as the arterialized blood will be mixed with considerably more venous blood in that location. In fact, the Sv̄O2 measured from the pulmonary artery does not appear to be affected at all by a dialysis fistula, as demonstrated in a case report in which the Sv̄O2 was noted to be the same when drawn off a pulmonary artery catheter with the fistula open or occluded (68.6% vs 68.3%).12 In our patient's case, the catheter tip was felt to be outside the right atrium, and we suspected a relatively greater impact from the dialysis fistula. Flow through the fistula was not measured. In situations like this, where the validity of the ScvO2 is in doubt, an alternative resuscitation end point should be considered. One such alternative is the serum lactate, which has been shown to perform as well as the ScvO2 in management of septic shock.13
Even in the cases of individuals with normal anatomy, clinicians need to be aware of other issues with ScvO2 monitoring.9 Because the tip of central venous lines typically lie in superior vena cava, the ScvO2 provides only information about the balance of oxygen delivery and consumption in the cephalad half of the body, and may not truly reflect the Sv̄O2, which takes into account venous return from the entire body. The depth to which the catheter is inserted may also affect the agreement between ScvO2 and Sv̄O2, with greater divergence between these values the further the CVC tip is from the right atrium.14 In addition, femoral venous oxygen saturation cannot be used as a surrogate for Sv̄O2, as these numbers can diverge widely.15 Although there is some disagreement, the available data suggest that, in shock states, ScvO2 overestimates the Sv̄O2 by 2–8%.14,16,17 The 2 parameters have been shown, however, to trend in the same direction in response to interventions or changes in clinical condition, and it is therefore important to focus on the trend in ScvO2 rather than the absolute value.18
Our patient's case demonstrates how certain patient-related factors may affect placement of a CVC and the utility of ScvO2 measurements. In particular, high ScvO2 readings may be obtained when the catheter tip is located downstream of an upper extremity dialysis fistula, especially when the tip is not at or below the caval-atrial junction. Providers placing these catheters to guide resuscitation in septic shock or ventilator management should be aware of these and other important limitations of the devices in order to avoid inappropriate patient management decisions.
Teaching Points
Ultrasound and pressure transduction should be used prior to insertion of a CVC to ensure venous, rather than arterial, placement. Placement should not proceed if there is any doubt regarding whether the needle or wire is in a central vein.
When questions persist about the location of the CVC after placement is complete and chest radiography is performed, simultaneous blood gases drawn off the CVC and from a known arterial site and pressure transduction can be used to confirm a venous location.
Care must be exercised when using the ScvO2 in patients with left-to-right shunts, including dialysis fistulas or anomalous pulmonary venous connection. In such situations clinicians should consider alternative resuscitation end points, such as serum lactate.
ScvO2 is a surrogate measure of Sv̄O2 and provides an assessment of the balance between oxygen delivery and oxygen consumption in the tissues. Because the ScvO2 overestimates Sv̄O2 in shock states, and agreement varies based on the distance of the catheter tip from the right atrium, clinicians should focus on the trends in these values rather than the absolute numbers.
Footnotes
- Correspondence: Andrew M Luks MD, Division of Pulmonary and Critical Care Medicine, Harborview Medical Center, 325 Ninth Avenue, Box 359762, Seattle WA, 98104. E-mail: aluks{at}u.washington.edu.
The authors have disclosed no conflicts of interest.
- Copyright © 2012 by Daedalus Enterprises Inc.