Abstract
Gorham syndrome is a rare disease that presents as progressive osteolysis, and may affect any part of the skeleton. The pathologic process involves the replacement of normal bone by aggressively expanding but non-neoplastic vascular tissue, resulting in massive osteolysis of the adjacent bone. If the spine and ribs are affected, the subsequent kyphosis and chest wall deformity may cause severe restrictive ventilatory impairment. We report a 34-year-old male with Gorham syndrome presenting as progressive kyphosis, severe back pain, unstable gait, and exertional dyspnea. Pulmonary function testing revealed severe restrictive ventilatory impairment. He underwent spinal surgery but could not be extubated after surgery. Postoperative left lower lung pneumonia and respiratory failure required prolonged mechanical ventilation. After a weaning program of pressure support ventilation and T-piece spontaneous breathing trials, he was successfully weaned from mechanical ventilation.
Introduction
Gorham syndrome is a rare chronic disorder that is characterized by the abnormal proliferation of thin-walled capillaries and small lymphatic vessels, resulting in massive osteolysis of the adjacent bone.1–3 It may affect any part of the skeleton, but most commonly involves the skull, shoulders, and pelvis.1–3 The vertebrae, ribs, and scapulae are other common sites.4 When the spine and ribs are involved, Gorham syndrome can lead to severe kyphosis and chest wall deformity,4,5 which cause severe restrictive ventilatory impairment. Gorham syndrome can occur at any age, but is common in adolescents and young adults.2
We saw a 34-year-old male with Gorham syndrome. He suffered from progressive kyphosis, unstable gait, exertional dyspnea, and severe restrictive ventilatory impairment. After spinal surgery he suffered respiratory failure and required prolonged mechanical ventilation, but was eventually extubated and weaned from mechanical ventilation.
Case Report
A 34-year-old male presented with lower back pain for the past 6 years. He was of normal intelligence and had no other clinical abnormalities. His family and medical histories were unremarkable. He consulted at the Department of Orthopedics, where x-ray and computed tomography revealed thoracolumbar junctional kyphosis and multiple osteolytic lesions. Computed-tomography-guided biopsy via the L3 vertebral body found granulation tissue with distinct vascularization. Gorham syndrome was diagnosed, based on clinical, radiologic, and histopathology features. He then received radiotherapy and interferon-α treatment.
Two years prior he had developed a left-side pleural effusion, and thoracentesis revealed chylothorax. He received pig-tail catheter insertion for drainage of the effusion. Subsequent pleurodesis with minocycline (400 mg in 30 mL normal saline) was successful, and there was no recurrent chylothorax. He continued to follow-up at the Department of Orthopedics.
Recently, he experienced progressive kyphosis with severe back pain and unstable gait. Reconstructed computed tomography of the thoracolumbar spine and chest revealed severe kyphosis and bone destruction at the T12-L1 level (Fig. 1) and left lower ribs (Fig. 2). On physical examination he had pronounced retraction of the left hemithorax and no palpable left lower ribs. He also had exertional dyspnea. Spirometry (CPFS/D USB, MGC Diagnostics, St Paul, Minnesota) revealed severe restrictive ventilatory impairment: FEV1 0.91 L (25% of predicted), FVC 0.94 L (21% of predicted), FEV1/FVC 0.97. Plethysmography (Dlite-Dx, MGC Diagnostics, St Paul, Minnesota) revealed a total lung capacity of 2.32 L (40% of predicted), residual volume of 1.2 L (88% of predicted), functional residual capacity of 1.69 L (53% of predicted), and a ratio of residual volume to total lung capacity of 0.52.
Reconstructed computed tomogram of the thoraco-lumbar spine shows severe kyphosis and bone destruction at the T12-L1 level.
Reconstructed computed tomogram of the chest shows destruction of the left lower ribs.
For the kyphosis he underwent surgery for T12 vertebral column resection, L1 pedical subtraction osteotomy with strut allografting, posterior instrumentation and infusion of T5-L4, and posterior decompression of T11-L1. He received preoperative education on incentive spirometry, directed cough, forced expiration, postural drainage and chest percussion, to prevent postoperative complications. However, postoperatively he became tachypneic, so extubation was not performed. He was then transferred to the ICU on pressure control ventilation with a pressure control level of 20 cm H2O, PEEP of 5 cm H2O, FIO2 of 0.30, and a tidal volume (VT) of around 450 mL.
He received fentanyl infusion (0.15 mg/h) continuously for his surgery wound pain. While at the ICU, mechanical ventilation was gradually tapered from pressure control ventilation to synchronized intermittent mandatory ventilation and pressure support ventilation (PSV). A T-piece spontaneous breathing trial was done after the pressure support level had been reduced to 8 cm H2O. On postoperative day 6 he could tolerate 30 min of T-piece spontaneous breathing trial, and the ventilator settings were VT 248 mL, breathing frequency 23 breaths/min, rapid shallow breathing index (ratio of breathing frequency to VT [RSBI]) 92.7, and maximal inspiratory pressure 18 cm H2O. Extubation was then performed.
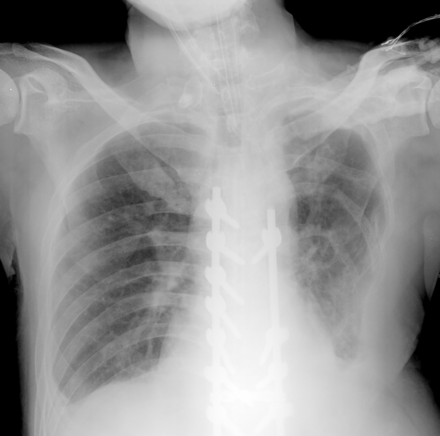
However, he continued to cough and had much sputum after extubation. Fever was then noted, and left lower lung pneumonia and bilateral pleural effusions were found via chest x-ray (Fig. 3). Diagnostic thoracentesis of the left pleural effusion revealed an exudate with protein 6.8 g/dL, glucose 123 mg/dL, and lactate dehydrogenase 288 IU/L. Left lower lung pneumonia with parapneumonic effusions was diagnosed. Right-side thoracentesis revealed clear fluid, and further analysis revealed transudate. His serum albumin was 1.9 g/dL, and the cause of the right-side pleural effusion was hypoalbuminemia. Parenteral antibiotics (subacillin 1.5 g [ampicillin sodium 1 g plus sulbactam sodium 0.5 g/vial] every 6 h) were prescribed because sputum culture revealed Acinetobacter baumannii complex. To manage the progressive tachypnea and shallow breathing, he received noninvasive ventilation. Because of progressive dyspnea with paradoxical respiration, use of accessory muscles, and desaturation (SpO2 90%) on noninvasive ventilation, he was re-intubated for mechanical ventilation on postoperative day 8. He then received pressure control ventilation, with pressure control 24 cm H2O, PEEP 6 cm H2O, FIO2 0.35, and VT of around 500 mL. Arterial blood gas analysis showed pH 7.48, PaO2 105 mm Hg, PaCO2 53 mm Hg, and HCO3– 35 mmol/L. Central venous pressure was 14 cm H2O (on PEEP 6 cm H2O). After reintubation, mechanical ventilation was gradually tapered to PSV. For the prolonged mechanical ventilation and difficult weaning of more than 2 weeks, he was transferred to the respiratory care center.
Chest x-ray after the first extubation attempt (post-operative day 6) shows left lower lung pneumonia, bilateral pleural effusions, and left lower ribs deformity. The endotracheal tube was in proper position.
At the respiratory care center, he was afebrile but mildly tachycardic (heart rate 110 beats/min), and tachypneic (breathing frequency 25–30 breaths/min) on PSV. He was alert but nervous. Laboratory examinations revealed hemoglobin 11.1 g/dL, white blood cell count 9.4 × 109/L, neutrophils 69%, and lymphocyte 15%. Liver and renal function tests, and electrolytes were within normal range. He received exercise training with hand cycle ergometer for 30–40 min once daily. As he became clinically stable, with SpO2 ≥ 90% on FIO2 ≤ 40%, PEEP ≤ 8 cm H2O, and no respiratory distress, the pressure support level was gradually tapered to achieve a breathing frequency of < 25 breaths/min and a VT of ≥ 5 mL/kg. When the pressure support level had been tapered to 8 cm H2O, he started to receive T-piece trials. The clinical assessment during T-piece trial was performed by continuously monitoring his consciousness level, respiratory pattern, SpO2, heart rate, breathing frequency, and blood pressure. The T-piece trial was terminated if there was agitation, depressed mental status, diaphoresis, cyanosis, increased accessory muscle activity, SpO2 < 90%, severe dyspnea, breathing frequency > 35 breaths/min or a 50% increase, heart rate > 140 beats/min or a 20% increase, systolic blood pressure > 180 mm Hg or a 20% increase, systolic blood pressure < 90 mm Hg, or cardiac arrhythmia.
After 15 min in the first T-piece trial his breathing frequency was 35 breaths/min and his heart rate was 130 beats/min. The duration of the T-piece trials were gradually increased as the patient tolerated them. After he tolerated a T-piece trial of 2 h he was screened daily to determine if he could be extubated. Initially, his ventilator settings were VT 228 mL, breathing frequency 28 breaths/min, RSBI 122.8, and maximal inspiratory pressure 15 cm H2O. After 10 days of exercise and T-piece training he passed the criteria for extubation (VT 268 mL, breathing frequency 27 breaths/min, RSBI 100.7, and maximal inspiratory pressure 24 cm H2O), and was extubated and liberated from mechanical ventilation on postoperative day 26. Figure 4 shows his chest x-ray on the day of extubation. He was then transferred to an ordinary ward for further care.
Chest x-ray before the second extubation attempt (post-operative day 26) shows left lower lung infiltration, bilateral pleural effusions, and left lower rib deformity.
Discussion
In 1955, Gorham and Stout defined a specific disease entity with idiopathic resorption of multiple bones.1 Gorham syndrome is a rare disease that presents as progressive osteolysis that can affect any part of the skeletal system, but most commonly the skull, shoulders, pelvis, vertebrae, ribs, and scapulae.1–4 Gorham syndrome has a slow, irregular, local progress, with concentric shrinkage of the shaft of bones, and tapering of the involved end, giving the appearance of “sucked candy.”2,3 Chylous pleural effusion is also a common presentation.4 The disease can occur at any age, but is common in adolescents and young adults. There is also no sex or racial predilection. The pathologic process is the replacement of normal bone by an aggressively expanding but non-neoplastic vascular tissue that results in the massive osteolysis of the adjacent bone.1–3 The etiology and pathogenesis of Gorham syndrome remain unknown.
Diagnosis is essentially one of exclusion, based on combined clinical, radiologic, and histopathology findings. During the acute phase, localized bone pain, swelling, progressive deformity, and contractures are common. Spontaneous fractures are likewise frequent. The end result is severe deformity and functional disability.1–3 Biochemical and hematologic tests are usually unremarkable and serve only to exclude other diagnoses.6 Treatments include radiation therapy, anti-osteoclastic medication, and interferon.7,8 If the disease becomes progressive, surgical resection of the lesion, with or without replacement by a prosthesis and bone graft, radiotherapy, and even amputation may be considered.7,8
Our patient had the typical presentation of Gorham syndrome: multiple osteolysis (radiologic features), bone destruction with granulation tissue, prominent vascularization (pathologic features), and chylothorax. Based on these, the diagnosis was made and the patient received radiotherapy, interferon, and surgery.
The literature regarding the influence of Gorham syndrome on the respiratory system is limited. Since Gorham syndrome often involves thoracic and spinal deformities, it is rational to conclude that Gorham syndrome influences respiratory mechanics with restrictive ventilatory impairment. Spinal surgery is sometimes necessary for various indications such as pain, deformity, and fracture. Because of impaired ventilation, such patients may be at risk of postoperative complications, particularly after vertebral reconstructive surgery.9 Moreover, impairment of chest wall mechanics is related to postoperative respiratory failure,10 which is related to increased chest wall work and respiratory energy expenditure.10,11 Thus, some patients may require intensive care after spine surgery.12 FVC < 40% of predicted is a sign of severe respiratory compromise after surgery.13
The severe restrictive disease is not just a challenge to anesthesia and surgical personnel. Respiratory complications are a major concern of spinal surgery, and include pneumonia, respiratory failure, prolonged postoperative intubation, and thromboembolic disease, among others.12 Factors that increase the risk of postoperative pulmonary complications include drugs, pain, trauma of the operation, decreased lung capacity, and decreased mobility.13 Thus, the prevention of complications is essential. Preoperative management and education on directed cough, forced expirations, postural drainage, and chest percussion are critical in preventing postoperative complications.14 Postoperative ventilatory problems lead to reintubation and prolonged mechanical ventilation, as in our patient, so extubation must be planned carefully.
Weaning a difficult-to-wean patient requires great skill and expertise.15 Clinical assessments are required to determine whether the patient is ready for reduction or removal of the ventilatory support.16 There are no specific weaning programs for patients with thoracic deformities, and our patient was weaned using a general weaning protocol. The 2 weaning strategies typically used are progressive reduction of ventilatory support, and progressively longer spontaneous breathing trials.15 Our patient initially received weaning via PSV, which is useful for overcoming the extra work of breathing imposed by the endotracheal tube, inspiratory valve, and ventilator circuit.17 PSV improves the efficacy of spontaneous breathing and reduces external respiratory work and oxygen consumption by the respiratory muscles.17
The initial pressure support level is usually determined by allowing the patient to breathe in a comfortable way.17 The suggested optimal initial pressure support level is the level that provides a breathing frequency 25–30 breaths/min and a VT of 10 mL/kg.17 The pressure support level is then decreased according to the patient's tolerance, usually by steps of 2–4 cm H2O twice a day.17 In general, tolerance of a pressure support of ≤ 8 cm H2O is required before attempting extubation.17
When our patient tolerated a pressure support level of 8 cm H2O, he was started on T-piece trials. Tolerance of a T-piece trial is a good test of the patient's capacity to maintain autonomous spontaneous breathing.15 The optimal T-piece trial duration is not well established, and 30 min to 2 h appears to provide a good balance between the fastest extubation time and the lowest reintubation risk.15 The evaluation of clinical tolerance of a T-piece trial includes signs of increased patient effort such as increased breathing frequency, accessory muscle use, paradoxical motion of the rib cage and abdomen, inappropriate recruitment of expiratory muscles, retraction of suprasternal and intercostal spaces, diaphoresis, cardiovascular instability, and abnormal mental status.15
Our patient was weaned twice, but with different results. Although the weaning protocols used were similar, the weaning conditions were different. The first time, mechanical ventilation was weaned rapidly after surgery. But he had operative wound pain, was on continuous intravenous fentanyl infusion, and had poor cough function. After extubation he developed pneumonia that required reintubation. On the second extubation attempt he was weaned gradually by tapering the mechanical ventilation to PSV and gradually lowering the pressure support level. His pneumonia was under control with antibiotics. However, after long-term mechanical ventilation and inactivity, his respiratory muscle strength and VT were decreased, compared to his initial measurements, so he received daily exercise and T-piece training. His maximal inspiratory pressure, VT, and cough function improved, and after passing the extubation criteria he was successfully extubated.
The benefits of exercise training in ventilated patients should be addressed. Critical illness has many devastating sequelae, including neuromuscular weakness and psychological and cognitive disturbances that frequently result in functional impairment.18 The inactivity of a ventilated patient impairs skeletal muscle, cardiovascular, and respiratory functions.19 Pulmonary rehabilitation for ventilated patients is emerging as an important strategy for preventing and treating acquired weakness.18 Greenleaf demonstrated the feasibility of exercise training in patients with bed-rest deconditioning syndrome and found that exercise training significantly improves muscular endurance.20 Chen et al also found that exercise training significantly improves pulmonary mechanics (VT and RSBI) and functional status in subjects with prolonged mechanical ventilation.21 Clini et al demonstrated that exercise training decreases breathing frequency, improves respiratory muscle strength, and increases lung volume.22 The mechanisms of the improvement from pulmonary rehabilitation are not precisely known. With increasing hyperinflation during exercise training, inspiratory muscle predominance may shift from the diaphragm to the accessory inspiratory muscles.23 Costi et al suggested improving physiologic factors such as aerobic capacity or ventilatory and peripheral muscle function.24
Footnotes
- Correspondence: Chou-Chin Lan MD PhD, Division of Pulmonary Medicine, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, 289 Jianguo Road, Xindian City, New Taipei City 23142, Taiwan. E-mail: bluescopy{at}yahoo.com.tw.
The authors have disclosed no conflicts of interest.
- Copyright © 2013 by Daedalus Enterprises