Introduction
Diffuse hemorrhage is a known but infrequent complication of amiodarone pulmonary toxicity and a cause of hemoptysis, with 12 cases reported up to now. Unlike other form of amiodarone pulmonary toxicity, early or very early1 onset seems to be common. It is thought to be mistaken for acute heart failure in many patients, due to similar clinical pattern and concomitant use of anti-aggregants or anticoagulants.2
Trepopnea refers to dyspnea that occurs in one lateral decubitus position. Trepopnea has been described in several conditions (Table 1), but there are no previous reports in patients with diffuse alveolar hemorrhage. We report a patient with hemoptysis and trepopnea due to amiodarone pulmonary toxicity, discuss characteristics of this infrequent complication of amiodarone treatment, and comment about the possible usefulness of trepopnea, if present, to suspect this condition.
Pathological Conditions in Which Trepopnea is Present
Case Summary
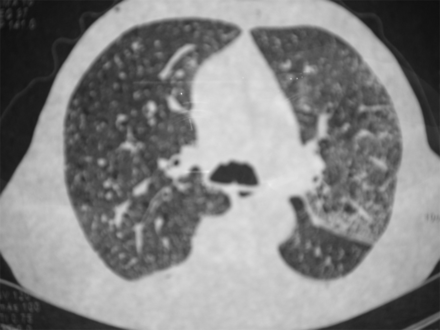
A 74-year old man was admitted to our unit because of dyspnea, hemoptysis, and anemia. The patient had a history of aortic regurgitation after biological prosthetic replacement, but he remained stable during the follow-up. He also suffered episodes of paroxysmal atrial fibrillation, controlled with bisoprolol and aspirin. Five months before admission, amiodarone and acenocoumarol were given because of another new episode of atrial fibrillation. The patient started with hemoptysis, a progressive feeling of dyspnea in the left hemithorax, and orthopnea in the right decubitus position. Chest x-ray showed an interstitial radiographic pattern (Fig. 1). Amiodarone was initially stopped for one month, which seemed to improve his condition. An out-patient pulmonary computed tomography confirmed interstitial involvement (Fig. 2), but it was interpreted as possible pulmonary edema. Then amiodarone was reintroduced and diuretics were given for the next 3 months. However, the patient had hemoptysis again, worsening of dyspnea, malaise, and persistent orthopnea in the right decubitus position.
Chest x-ray one month after starting amiodarone shows interstitial pattern.
Computed tomogram one month after starting amiodarone show interstitial involvement in both lungs.
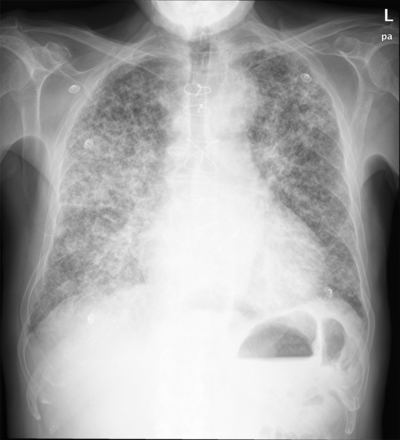
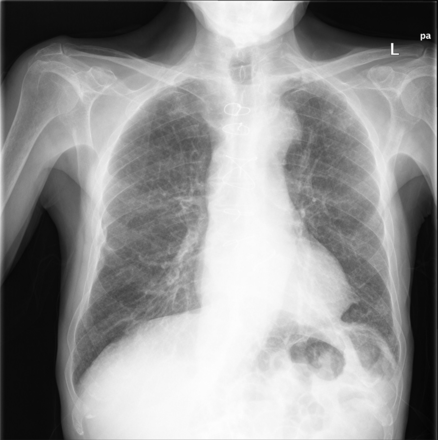
Thus, he was finally admitted. At examination, he was pale, dehydrated, and cachectic. Although his breath rate was normal, his SpO2 was 84%. There were pulmonary crackles but no cyanosis, augmented venous pressure, third tone, or edema. Blood test showed severe anemia and excessive hypocoagulation. Chest x-ray revealed prominent infiltrates (Fig. 3). Details on laboratory tests are provided in Table 2. The patient improved with oxygen, transfusion, and fluids. Anticoagulation was effectively reverted by means of vitamin K and definitively stopped. However, after 8 days of admission, trepopnea, hemoptysis, and infiltrates persisted. A bronchoscopy demonstrated exudative hemorrhagic alveolar damage consistent with drug toxicity. Amiodarone was stopped and prednisone was started at a daily dose of 30 mg. After 3 months, clinical and radiographic recovery was complete (Fig. 4).
Chest x-ray at admission shows prominent alveolo-interstitial infiltrates.
Laboratory Tests
Chest x-ray after 3 months of therapy shows the infiltrates have disappeared.
Discussion
We report a case of trepopnea associated with amiodarone-induced diffuse alveolar hemorrhage. An early accumulative dose of 6 g was sufficient to cause interstitial disease, but hemoptysis with severe anemia and respiratory failure arose at 24 g. The most known side effect of amiodarone use is chronic fibrosing alveolitis. Acute lung toxicity is rare, commonly taking the form of an acute or subacute interstitial pneumonitis, but early alveolar hemorrhage has been also reported. Diffuse hemorrhage may lead to misdiagnosis. The symptoms and radiographic findings were initially misinterpreted as acute heart failure in this case. When trepopnea is present in patients with heart failure, it usually occurs in the left recumbent position and they prefer the right lateral decubitus position. The patient lies with the more affected lung down. It seems a self-protecting mechanism to augment cardiac output and to attenuate the imbalance of cardiac autonomic nervous activity.3 Inversely, this patient had dyspnea in the left, which could help to differentiate acute heart failure from other conditions. Computed tomography scan (see Fig. 1) seemed to show the left lung more affected. Similarly, asthenia, mild fever, and dyspnea may be mistaken for cancer or infection. Of note, the influence of coexistent anticoagulants has been noted as a problem in all of the previous reports. The temporal sequence of data suggests that amiodarone was the essential cause in this case. The probability of an adverse drug reaction can be measured by the Naranjo et al scale.4 Briefly, it consists of 7 items: temporal sequence, known adverse drug reaction, alternative explanation, adverse drug reaction evidence, laboratory tests, improvement after withdrawal, and worsening with reintroduction. The likelihood of a drug reaction is improbable when the score is < 1 point, possible when the score is 1–4 points, probable when the score is 5–8 points, and definitive when the score is 9–10. This case fulfilled 9 points for amiodarone. The findings detected by computed tomography suggested initial amiodarone toxicity in the interstitium, before the alveolar damage, which could not be attributed to anticoagulation. In addition, whereas acenocoumarol was maintained, the first discontinuation of amiodarone was followed by improvement until reintroduction. Although anticoagulation was reverted at admission, symptoms continued. Otherwise, previous antiplatelet therapy was given without hemoptysis. Histology showed exudative changes, and prescription of prednisone treated the condition.
Teaching Points
Amiodarone-induced pulmonary hemorrhage is a rare complication associated with dyspnea, hemoptysis, and asthenia, which may be overlooked in patients with heart disease. Trepopnea may be a useful symptom to differentiate pulmonary disease from heart failure when the patient prefers the left decubitus position.
Footnotes
- Correspondence: Luis Miguel Blasco Mata, Unidad de Alta Resolución Hospitalaría, Hospital Universitario Marqués de Valdecilla, Avenida Marqués de Valdecilla, 39008 Santander, Cantabria, Spain. E-mail: grullus99{at}yahoo.es
The author has disclosed no conflicts of interest.
- Copyright © 2013 by Daedalus Enterprises