Abstract
BACKGROUND: Early identification of treatment failure for nosocomial pneumonia remains a major challenge. The goal of this study was to test whether procalcitonin kinetics can be used to assess the clinical efficacy in older critically ill patients with nosocomial pneumonia.
METHODS: A prospective observational study was conducted with 60 subjects (≥ 65 y old) admitted to the ICU with severe nosocomial pneumonia. Serum procalcitonin was measured on days 0, 3, and 7 and at the end of treatment. The procalcitonin time course was analyzed according to the therapeutic efficacy.
RESULTS: Procalcitonin levels were elevated in all subjects (n = 60) on day 0, and the median level (range) was 2.5 (0.85–42.7) μg/L. There were no differences in procalcitonin between the improved subjects (n = 41) and those without improvement (n = 19) on day 0 (P > .05). However, lower procalcitonin levels on days 3 and 7 and at the end of treatment (all P < .05) and greater rates of procalcitonin decline between days 0 and 3 (ΔPCTd3%; 29.5 ± 10.8% vs 15.1 ± 5.9%, P = .009) were observed in the improved subjects compared with those with no improvement. ΔPCTd3% was the best single predictor of efficacy (area under the curve of 0.79, P < .001) and had a sensitivity of 75.7% and a specificity of 72.0% with a threshold of 26.2%. By comparison, traditional parameters and absolute procalcitonin failed to predict treatment response (P > .05). Indeed, the combination of ΔPCTd3% > 26.2% and a modified Clinical Pulmonary Infection Score of < 6 points improved the predictive value (area under the curve of 0.89, sensitivity of 81.3%, specificity of 86.5%).
CONCLUSIONS: Procalcitonin levels were not influenced by aging, and procalcitonin kinetics might help to identify treatment failure. ΔPCTd3% in combination with the Clinical Pulmonary Infection Score has been shown to be a marker of clinical efficacy at an earlier stage.
- nosocomial pneumonia
- procalcitonin
- procalcitonin kinetics
- therapeutic efficacy
- modified Clinical Pulmonary Infection Score (mCPIS)
- older critically ill patients
Introduction
Older critically ill patients clearly face an increased risk for nosocomial infection. Nosocomial pneumonia remains one of the major causes of morbidity and mortality.1,2 Re-evaluation at 72 h after treatment is crucial to detect treatment failure as well as to prevent other complications.3,4 Unfortunately, traditional parameters fail to assess the clinical efficacy at an earlier stage. For example, fever and leukocytosis are generally considered to lack specificity5; sputum cultures are time-consuming and create difficulties in differentiating colonization from infection6; and chest radiography also remains a challenge in older patients because inflammatory absorption, if any, often becomes evident only during the late phase of infection and is confused with non-infection factors (eg, pulmonary edema).7 The lack of effective measures to monitor therapeutic efficacy has led to efforts aimed at biomarkers.
Procalcitonin, a 116-amino acid polypeptide and a precursor of calcitonin, was first described by Assicot et al8 as a marker of bacterial infection. Previous studies9–11 have indicated that procalcitonin is superior to other commonly used parameters in its specificity for bacterial infection and might help to guide antibiotic stewardship in lower respiratory tract infections. However, its role in critically ill patients is still disputed.12–14 In particular, little is known about the early time-dependent changes in procalcitonin and its behavior in older patients. Studies in emergency departments and in older patients with community-acquired pneumonia or COPD exacerbation are sparse and provide conflicting results.15–18 To date, the utility of procalcitonin for predicting therapeutic efficacy at an earlier stage has not been assessed in older patients admitted to the ICU and requires further study.
QUICK LOOK
Current knowledge
Nosocomial pneumonia is a major cause of morbidity and mortality in elderly patients. Re-evaluation at 72 h is important to escalate or de-escalate therapy and define treatment failures. Clinical markers of treatment failure are subject to interpretation, and clear criteria are not available.
What this paper contributes to our knowledge
Procalcitonin is a marker of infection, and, the rate of decline, combined with the Clinical Pulmonary Infection Score, might help identify treatment failure at 72 h. This study found that procalcitonin over the first 3 d of treatment was not altered by age, but procalcitonin alone could not be used to guide antibiotic therapy.
Methods
Study Setting and Population
Older patients (≥ 65 y old) admitted to our 18-bed mixed medicosurgical ICU from January 2009 to June 2010 with suspected nosocomial pneumonia were eligible. Patients were excluded if they had a diagnosis of immunosuppression, non-infection-induced agranulocytosis, confirmed non-bacterial infection, concomitant infections at other sites on admission, non-microbiologically confirmed nosocomial pneumonia, non-bacterial nosocomial pneumonia, progressive infection at other sites, or incomplete determination of procalcitonin with uncertain therapeutic effects due to duration of treatment of < 5 d; or incomplete clinical data at the final analysis.
This study was approved by the ethics committee of our hospital, and the board waived informed consent because of the non-intervention design and retrospective group.
Baseline Assessment and Data Collection
The recorded data included age, sex, comorbid conditions, patient classification (medical or surgery), and prior hospitalization before ICU admission. The following data were obtained on days 0, 3, and 7 and at the end of treatment: white cell count; body temperature; sputum culture results; procalcitonin levels; chest radiography and other laboratory tests required to calculate the modified Clinical Pulmonary Infection Score (mCPIS),19 the Acute Physiology and Chronic Health Evaluation II score,20 and the Sequential Organ Failure Assessment score21; the presence or absence of infection at other sites; appropriate antimicrobial treatment (the drug had an in vitro activity against the isolated strain); duration of mechanical ventilation; ICU stay; and 28-d ICU crude mortality.
Study Design
This was a prospective observational study, and treatment decisions were left up to clinicians. On admission, all subjects immediately received empirical antimicrobial therapy. After that, modifications were according to culture results and treatment response. The duration of treatment was continued for at least 8 d in subjects with uncomplicated pneumonia and for at least 2–3 weeks (with decisions made according to therapeutic response and severity of illness) in those with concomitant bacteremia or multidrug-resistant (MDR) infection (defined as resistance to ≥ 3 classes of antimicrobial agents, including cephalosporins, aztreonam, carbapenems, aminoglycosides, fluoroquinolones).4 Mechanical ventilation and airway management were performed in accordance with a standard protocol.22
Microbiologic Processing and Procalcitonin Assay
Respiratory samples were obtained by tracheal aspiration, bronchoalveolar lavage, or sputum specimen. Only good-quality specimens (< 10 epithelial cells and > 25 white blood cells per low-power field) were cultured, and more than 3+ of bacterial growth using a semiquantitative culture method was considered positive.23 Procalcitonin concentrations were measured using a commercially available immunoluminometric assay system (Brahms Diagnostica, Hennigsdorf bei Berlin, Germany). Functional assay sensitivity was defined as the lowest value with an interassay coefficient of variance < 20% of 0.1 μg/L. The rate of change in procalcitonin (ΔPCT%) was calculated using the following formula: ΔPCTdx% = (PCTd0 − PCTdx)/PCTd0 × 100. Values ≤ 0 indicate decreasing procalcitonin concentrations. Conversely, values ≤ 0 indicate unchanged or increasing procalcitonin concentrations. All assays were performed according to the manufacturer's instructions and standard microbiology guidelines.23,24
Definitions and Outcome Measures
Nosocomial pneumonia was suspected when a patient who was in the hospital or residing in a long-term care facility (> 48 h) or who presented < 7 d after hospital discharge with an initial hospitalization of ≥ 3 d developed a new and persistent radiographic infiltrate plus 2 of the following: body temperature > 38°C or < 36°C, white blood cell count > 11,000 cells/μL or < 4,000 cells/μL, and a macroscopically purulent tracheal aspirate.25
Diagnosis of severe nosocomial pneumonia was defined using American Thoracic Society guidelines25 and met any one of the following conditions: (1) shock defined as systolic blood pressure of < 90 mm Hg or diastolic blood pressure of < 60 mm Hg; (2) respiratory failure (ie, mechanical ventilation or the need for an FIO2 of > 0.35 to maintain an oxygen saturation of > 90%); (3) requirement of vasopressor therapy for > 4 h; (4) urine output of < 20 mL/h or total urine output of < 80 mL/h for > 4 h, unless oliguria was present due to a condition other than infection/sepsis; (5) acute renal failure requiring dialysis; or (6) rapid radiographic progression, multilobar pneumonia, or cavitation of a lung infiltrate.
Clinical efficacy was assessed within 5 d of the end of treatment or the 3rd week of antimicrobial treatment by investigators and categorized as clinical improvement (complete or partial resolution of signs and symptoms of pneumonia) or no improvement (deterioration or no improvement of signs and symptoms).25 Microbiologic responses were classified as eradication (including presumed microbiologic eradication) or persistence (including presumed microbiologic persistence) based on culture results and clinical responses.
Statistical Analyses
SPSS 13.0 (SPSS, Chicago, Illinois) was used for all statistical analyses. Normally distributed parameters were expressed as mean ± SD and compared using the Student t test. Non-normally distributed parameters were expressed as median (range) and analyzed using the Mann-Whitney U test. Unordered categorical variables were expressed as percentages, and the difference was analyzed using the chi-square test. Logistic regression analysis was used to analyze the influential factors of clinical efficacy. The predictive ability was estimated by using receiver operating characteristic curves. All tests were 2-tailed, and P values < .05 were considered statistically significant.
Results
Subject Characteristics
During the study period, of 291 ICU patients (≥ 65 y old), 107 had suspected nosocomial pneumonia, and 47 were excluded. The reasons for exclusion are listed in Figure 1. A total of 60 subjects were included. The mean age was 70.7 ± 12.7 y. The group was 71.7% male, and 38.3% were surgical subjects. Forty-one subjects were categorized as having clinical improvement, and 19 as no improvement.
Study flow chart. PCT = procalcitonin.
The subject characteristics were as follows: 32 pre-ICU-acquired cases of nosocomial pneumonia and 28 ICU-acquired cases of nosocomial pneumonia, multilobar pneumonia in 25% of subjects (n = 15), concomitant bacteremia in 16.7% of subjects (n = 10), septic shock in 31.7% of subjects (n = 19), and dysfunction of > 2 organs in 55.0% of subjects (n = 33). Invasive ventilation was used in 56 subjects (93.3%, 20 diagnosed as having ventilator-associated pneumonia). The Acute Physiology and Chronic Health Evaluation II score and mCPIS on admission were 15.8 ± 6.8 and 7.1 ± 1.5, respectively. The duration of antimicrobial treatment was 17.2 ± 5.5 d, and 28-d ICU crude mortality was 8.3% (5 cases with MDR infections died). Variables are summarized in Table 1.
Clinical Characteristics of 60 Subjects With Confirmed Bacterial Nosocomial Pneumonia
Serum Procalcitonin Concentrations and Influential Factors
Procalcitonin concentrations were measured in 365 samples taken from eligible patients and were analyzed in 240 samples from enrolled subjects. Procalcitonin was elevated (> 0.5μg/L) in all subjects on day 0, and the median level (range) was 2.5 (0.85–42.7) μg/L. Procalcitonin levels were associated with appropriate empirical antibiotic therapy (r = 0.55, P = .045), age (r = 0.55, P = .045), septic shock (r = 0.78, P = .003), and organ dysfunction (r = 0.69, P = .005) as assessed by the Sequential Organ Failure Assessment score, but sex (r = 0.07, P = .60), serum creatinine concentration (r = 0.10, P = .89), surgery (r = 0.50, P = .07), MDR infection (r = 0.08, P = .72), type of nosocomial pneumonia (eg, ventilator-associated pneumonia; r = 0.16, P = .59), mixed bacterial infection (r = 0.20, P = .45), microbiologic eradication (r = 0.21, P = .27), therapeutic efficacy (r = 0.09, P = .78), and prognosis (r = 0.26, P = .43) were not. Septic shock (odds ratio [OR] 5.1, 95% CI 3.5–10.5, P = .01) and organ dysfunction (OR 3.2, 95% CI 1.1–8.7, P = .03) were independent predictors of higher procalcitonin concentrations upon multivariable analysis.
Procalcitonin Kinetics and Its Role in Predicting Clinical Efficacy
There were no differences between the subjects with improvement and those without with regard to procalcitonin on day 0 (3.8 ± 2.0 vs 4.2 ± 2.3 μg/L, P = .29). However, procalcitonin concentrations on day 3 (3.2 ± 0.7 vs 4.0 ± 1.8 μg/L, P = .040), on day 7 (1.5 ± 0.6 vs 3.7 ± 1.7 μg/L, P = .004), and at the end of treatment (0.6 ± 0.3 vs 2.5 ± 1.3 μg/L, P = .001) were lower in improved subjects. By comparison, the rate of procalcitonin decline between days 0 and 3 (ΔPCTd3%) was significantly faster in the subjects with improvement compared with those without (29.5 ± 10.8% vs 15.1 ± 5.9%, P = .009). In contrast, leukocytosis, temperature, granulocyte percentage, and mCPIS did not differ between the 2 groups (Fig. 2).
Kinetics of clinical parameters and procalcitonin concentrations in the improved subjects and in those with no improvement from day 0 to the end of treatment. The solid line denotes the improved group, and the dotted line indicated the group with no improvement. Asterisks denote statistical significance between groups. Results are expressed as median values with the interquartile range (25–75%). WBC = white blood cell; mCPIS = modified Clinical Pulmonary Infection Score.
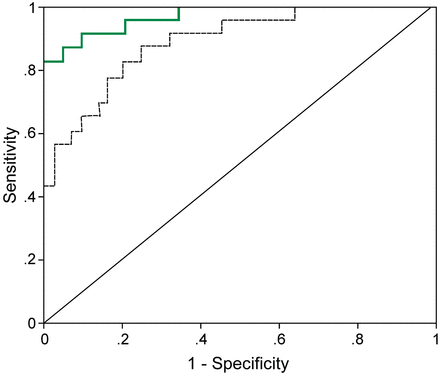
PCTd3% had the closest correlation with therapeutic efficacy (area under the curve of 0.791, 95% CI 0.696–0.895, P < .001) compared with mCPIS on day 3 (area under the curve of 0.683, 95% CI 0.529–0.719, P = .048), white blood cell count (area under the curve of 0.238, P = .75), granulocyte percentage (area under the curve of 0.429, P = .63), body temperature (area under the curve of 0.452, P = .19), and absolute procalcitonin concentration (area under the curve of 0.540, P = .79). The ΔPCTd3% cutoff value of 26.2% had a sensitivity of 75.7% and a specificity of 72.0% for predicting clinical improvement. Combining the values for ΔPCTd3% (> 26.2%) and mCPIS (< 6 points) together increased the area under the curve to 0.890, sensitivity to 81.3%, and specificity to 86.5%, which was significantly better than ΔPCTd3% alone (P < .05) (Fig. 3).
Shown are the receiver operator characteristic curves for procalcitonin kinetics within the first 72 h of treatment (rate of procalcitonin decline on day 3 [ΔPCTd3%; dotted line] and ΔPCTd3% combined with the modified Clinical Pulmonary Infection Score [mCPIS; green line]) on day 3. For ΔPCTd3% > 26.2% and mCPIS < 6 points, the area under the curve was 0.890, sensitivity was 81.3%, and specificity was 86.5%.
Serum Procalcitonin Concentration and Microbiologic Response
A total of 75 strains of bacteria (45 MDR strains) were isolated, including 20 strains of Acinetobacter baumannii (18 MDR strains); 16 of Pseudomonas aeruginosa (10 MDR strains); 10 of methicillin-resistant Staphylococcus aureus; 6 of Stenotrophomonas maltophilia (all MDR); 5 each of Klebsiella pneumoniae and Escherichia coli; 4 of Serratia marcescens; 3 of methicillin-sensitive S. aureus; and 2 each of Proteus mirabilis, Haemophilus influenzae, and Enterococcus faecalis (repeated culture-positive from subjects with recurrent aspiration, one MDR strain).
Subjects in the microbiologic eradication group (n = 15) compared with the microbiologic persistence group (n = 45) had a lower incidence of MDR infection (33.3% [5 subjects] versus 55.6% [25 subjects], P = .003) and lower procalcitonin concentrations on day 3 (2.5 ± 1.3 vs 4.1 ± 2.1 μg/L, P = .03), day 7 (0.9 ± 0.5 vs 3.8 ± 1.8 μg/L, P = .001), and at the end of treatment (0.3 ± 0.1 vs 1.5 ± 1.1 μg/L, P < .001), but similar levels on day 0 (3.9 ± 1.8 vs 4.3 ± 2.1 μg/L, P = .27). In the microbiologic persistence subgroup, ΔPCTd3% was also clearly higher in the clinically improved subjects (n = 26) compared with the subjects without improvement (n = 19): 25.6 ± 7.8% vs 15.1 ± 5.9% (P = .02).
Influential Factors of Clinical Efficacy
In contrast to the improved subjects, the subjects without improvement were significantly older (75.1 ± 14.0 vs 69.5 ± 7.3 y, P < .05) and had higher Sequential Organ Failure Assessment scores (10.5 ± 7.3 vs 9.5 ± 6.8, P < .05), prolonged antimicrobial treatment (18.5 ± 5.8 vs 15.3 ± 4.7 d, P < .05) and duration of septic shock (7.1 ± 3.7 vs 4.8 ± 2.5 d, P < .05), more frequent underlying cardiac dysfunction (47.4% vs 14.6%, P < .05), concomitant bacteremia (21.1% vs 14.6%, P < .05), multilobar pneumonia (36.8% vs 19.5%, P < .05), septic shock (36.8% vs 24.5%, P < .05), dysfunction of ≥ 2 organs (68.4% vs 48.8%, P < .05), and inappropriate antibiotic therapy (52.6% vs 17.1%, P < .05). There were no differences between groups regarding sex, prior hospitalization before ICU admission, mCPIS, proalbumin concentrations, body temperature, leukocytes, granulocyte percentage, mixed bacterial infection, and subject classification (P > .05 for each) (see Table 1). In a multiple logistic regression analysis, underlying cardiac dysfunction (OR 5.3, 95% CI 2.1–9.8, P = .02), multilobar pneumonia (OR 3.4, 95% CI 1.3–7.5, P = .03), inappropriate empirical antibiotic therapy (OR 5.1, 95% CI 1.1–8.5, P = .02), concomitant bacteremia (OR 2.6, 95% CI 1.6–7.8, P = .03), dysfunction of ≥ 2 organs (OR 2.3, 95% CI 1.5–15.2, P = .03), and ΔPCTd3% < 26.2% (OR 8.2, 95% CI 3.3–11.75, P = .01) were independent predictors of treatment failure.
Discussion
Procalcitonin and its kinetics as a way to assess clinical efficacy at an earlier stage were addressed in older patients with nosocomial pneumonia. Our findings suggest that procalcitonin kinetics within the first 72 h of sepsis management may be a useful tool for predicting treatment failure and might provide an opportunity to modify antibiotics and improve outcomes.
First, elevated procalcitonin levels were observed in all subjects with severe bacterial nosocomial pneumonia and were also observed to be associated with severity of illness, whereas the type of pneumonia, MDR infection, or renal function were not. These findings suggest that procalcitonin kinetics may not be influenced by aging and serve as an indicator of the severity of bacterial infection. The procalcitonin release mechanism may be one explanation for this: procalcitonin originates from various tissues and cells (eg, fat, liver, stomach, lung), and its level was in this study strongly associated with the degree and extensity of sepsis, but specific bacterial strains, source of infection, and impaired renal function were not.9,26–29 Indeed, a relatively high procalcitonin level was used in comparison with a previous study that used a procalcitonin concentration of ≥ 0.38 μg/L for diagnosis of bacterial infection in older subjects.17 Differences in the case mix (a higher proportion of surgical subjects were included) and critical severity (a higher proportion of septic shock and higher Sequential Organ Failure Assessment scores) may contribute to higher procalcitonin concentrations.30–32
Second, procalcitonin kinetics within the first 72 h was also found to be significantly different because a decrease of ≥ 26.2% was expected in the improved subjects and was regarded as an independent predictor of clinical efficacy. As a result, patient management might be reassessed if procalcitonin levels fall slowly (such as < 26.2% between days 0 and 3). In such cases, modification of the initial empirical therapy may be considered while the microbiologic findings, if any, are still pending. In contrast, absolute procalcitonin failed to predict clinical efficacy due to the fact that it was affected by various factors, including concurrent infection at other sites, severity of infection, trauma, and surgery.33 Similarly, use of mCPIS is also not suitable for patients with trauma, surgery, or ARDS,34,35 but better predictive ability was observed when combined with procalcitonin kinetics.
Interestingly, procalcitonin kinetics indicting microbiologic eradication (but not serving as a marker of clinical efficacy) helped to distinguish between improved subjects and those with no improvement. This result was consistent with the experimental data: the procalcitonin time course is thought to be closely dependent on bacterial load and the host response to microbial challenge.36 Procalcitonin kinetics may offer a solution for the dilemma regarding positive sputum cultures (interpretation of colonization or infection) and guide in determining the appropriate duration of antibiotics.
In fact, few studies conducted with ICU patients have demonstrated a relationship between early time-dependent changes in procalcitonin and therapeutic efficacy. We found that the rate of change in procalcitonin in the improved subjects from day 0 to the end of treatment (90.1 ± 20.7%) was similar to published data on a procalcitonin-guided de-escalation algorithm (antibiotics were stopped if procalcitonin fell by > 80% of its peak value).12,14 Additional studies are required to estimate the exact threshold of procalcitonin for predicting therapeutic efficacy at an earlier stage.
Limitations
We are aware of the limitations of our study. First, the type of study (single-center) and small sample size restricted further subgroup analysis (such as ventilator-associated pneumonia and MDR infection). Second, the lack of an accepted standard for diagnosis of nosocomial pneumonia and the enrollment of only subjects with microbiologically confirmed nosocomial pneumonia may have led to selection bias. Third, we could not determine whether the predictive value of ΔPCTd3% is equally applicable to very old individuals due to few patients who are > 90 y old. Finally, whether the ideal strategy involves the use of procalcitonin kinetics in sepsis management in this population remains to be established.
Conclusions
In older critically ill subjects with nosocomial pneumonia, procalcitonin and its kinetics were not influenced by aging, and they might help to identify therapeutic efficacy at an earlier stage. The rate of procalcitonin decline within the first 72 h in combination with mCPIS has been shown to be an early marker of clinical efficacy. Further studies are needed to assess the utility of the daily monitoring of procalcitonin in addition to clinical evaluation during the early management of sepsis.
Footnotes
- Correspondence: Yan Shi MD, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, 1 Shuai Fu Yuan Wang Fujing Street, Dongcheng District, Beijing 100730, China. E-mail: pumchshi{at}sina.com.
The authors have disclosed no conflicts of interest.
- Copyright © 2014 by Daedalus Enterprises