Abstract
BACKGROUND: Forced expiration may assist secretion movement by manipulating airway dynamics in patients with cystic fibrosis (CF). Expiratory resistive breathing via a handheld incentive spirometer has the potential to control the expiratory flow via chosen resistances (1–8 mm) and thereby mobilize secretions and improve lung function. Our objective was to explore the short-term effect of using a resistive-breathing incentive spirometer on lung function in subjects with CF compared with the autogenic drainage technique.
METHODS: This was a retrospective study. Subjects with CF performed 30–45 min of either the resistive-breathing incentive spirometer (n = 40) or autogenic drainage (n = 32) technique on separate days. The spirometer encourages the patient to exhale as long as possible while maintaining a low lung volume. The autogenic drainage technique includes repetitive inspiratory and expiratory maneuvers at various tidal breathing magnitudes while exhalation is performed in a sighing manner. Spirometry was performed before and 20–30 min after the therapy.
RESULTS: Use of a resistive-breathing incentive spirometer improved FVC and FEV1 by 5–42% in 26 subjects. The forced expiratory flow during the middle half of the FVC maneuver (FEF25-75%) improved by >20% in 9 (22%) subjects. FVC improved the most in subjects with an FEV1 of 40–60% of predicted. Improvements negatively correlated with baseline percent-of-predicted FVC values provided improvements were above 10% (r2 = 0.28). Values improved in a single subjects using the autogenic drainage technique.
CONCLUSIONS: These 2 techniques may allow lower thoracic pressures and assist in the prevention of central airway collapse. The resistive-breathing incentive spirometer is a self-administered simple method that may aid airway clearance and has the potential to improve lung function as measured by FVC, FEV1, and FEF25-75% in patients with CF.
Introduction
Cystic fibrosis (CF) is a disorder that interrupts the lungs' normal mucus secretion, causing excessive production of viscid mucus, which leads to mucus plugging, recurrent infections, and inflammation, followed by airway damage and lung function deterioration.1–3 Airway clearance therapy aims to improve mucociliary clearance through the removal of tenacious obstructing mucus from the airways, thus improving ventilation.3–5 Indeed, Cochrane reviews1,5–7 have concluded that airway clearance techniques have the short-term effect of increasing mucus transport, and there appears to be no advantage of either conventional chest physiotherapy or oscillating devices over other airway clearance techniques in the primary outcome measure of lung function.
Autogenic drainage to mobilize secretions8 is a respiratory self-drainage technique that uses controlled tidal breathing performed at different lung volume levels. In this technique, the velocity or force of the expiratory air flow must be self-adjusted at each level of inspiration so that the highest possible air flow is reached in that generation of bronchi without causing airway collapses during coughing. Although the autogenic drainage technique was found to be useful in mobilizing and diluting secretions and improving oxygen saturation, lung function improvements could not be demonstrated.6
Handheld devices using expiratory air-flow exercises through fixed resistance may motivate patients to increase self-management of their condition.9–14 Such devices include oscillatory positive expiratory pressure (PEP) devices, which produce air-flow oscillations in addition to PEP. Studies have shown that the PEP technique improved lung volumes and expiratory flow when used over a 10–12-month period, but no immediate effect on lung function was found.9–14
A handheld incentive spirometer is designed to encourage patient inhalation by slow deep breaths while providing the patient with information that helps maintain an inhaled lung volume. This device, without the resistance, is widely used after upper abdominal operations accompanied by postoperative pulmonary complications, and has been shown to improve sputum expectoration.15,16 An advanced incentive spirometer includes the possibility of performing long expirations against a chosen resistance level (resistive-breathing incentive spirometer). Thus, it may be used for respiratory physical therapy in patients with CF. The aim of this study was to explore the effect of using a resistive-breathing incentive spirometer for airway clearance by considering the short-term effect on lung function in subjects with CF.
QUICK LOOK
Current knowledge
Cystic fibrosis (CF) interrupts the lungs' normal mucus secretion, causing excessive production of viscid mucus, which leads to mucus plugging, recurrent infections, and inflammation, followed by airway damage and lung function deterioration. Airway clearance therapy aims to improve mucociliary clearance, lung function, and quality of life. To date, research has shown that airway clearance techniques have the short-term effect of increasing mucus transport, and there appears to be no advantage of either conventional chest physiotherapy or oscillating devices over other airway clearance techniques.
What this paper contributes to our knowledge
This retrospective study of expiratory resistive breathing via a handheld incentive spirometer compared with autogenic drainage in subjects with CF demonstrated improved lung function following resistive-breathing incentive spirometer use. Both FVC and FEV1 improved in more than half of subjects studied. These changes are temporal, and the impact on long-term outcomes are not known.
Methods
Subjects
Data were acquired from subjects with CF who routinely visited the ambulatory clinic in our center. The diagnosis of CF was made by the presence of at least one typical clinical manifestation of the disease combined with an abnormal sweat test (chloride >60 mmol/L) and/or the presence of CF mutations.
We recruited subjects with CF (>8 y old) who visited the CF clinic for their routine follow-ups (once in 2–3 months) over 1 y, and we performed pulmonary function tests before and 20–30 min after the therapy session. Subjects in a stable clinical condition had to be familiar with pulmonary function tests and respiratory therapy clearance techniques. Patients who were on oxygen or home intravenous therapy or who had current pulmonary exacerbations or hospitalizations were excluded. Otherwise, the study did not require changes in medication or lifestyle. All participants were familiar with the use of the autogenic drainage technique for airway clearance as a daily treatment, but not with the resistive-breathing incentive spirometer, which was introduced in the physiotherapy session. The Helsinki Board of Sheba Medical Center approved the study (5583-08-SMC).
Study Design
This was a retrospective study performed from 2008 to 2011. All data selected for inclusion in this retrospective study were obtained from subjects who performed spirometry before and after airway clearance therapy, regardless of the technique applied. All subjects performed the autogenic drainage technique regularly on clinic visits. The resistive-breathing incentive spirometer technique was introduced to all subjects, and most elected to continue performing it on a regular basis in clinical visits during the study period. We sought to compare the effect of every intervention on spirometry measurements for each subject on both techniques.
The physiotherapy sessions did not require changes in medication or life style. After initially performing a baseline lung function test, subjects underwent a respiratory exercise session using one of the 2 methods (resistive-breathing incentive spirometer or autogenic drainage). Twenty to 30 min after physiotherapy ceased, the lung function test was repeated. Both respiratory therapy sessions were coached by the same respiratory therapist.
Interventions
The Tri-Gym (Koo Asia, Tsuen Wan, Hong Kong) (Fig. 1) is a handheld volumetric breathing exerciser consisting of 3 graduated cylinders, each containing a differently colored ball. The volumetric target goals are 600, 900, and 1,200 mL/s. A corrugated tube with a mouthpiece is connected to the superior lateral part of the device. An adjustable valve with tiny openings from 1 to 8 mm in diameter is placed at the opening of the device to control the expiratory breathing resistance.
The Tri-Gym handheld device.
With a resistive-breathing incentive spirometer, the breathing maneuvers are similar to those used in the autogenic drainage technique, but the breathing effort is resistance-dependent. Breathing tasks were performed in a sitting position. Subjects held the breathing device in a position that allowed expiratory resistive breathing. They were then asked to seal their mouths around the mouthpiece. When relaxed, subjects were asked to inhale to total lung capacity and exhale as much as possible once or twice to the point where tidal breaths at low lung volume could be performed against a chosen resistance. The level of resistance was selected in relation to the ability of each subject to maintain the volumetric ball at a target volume for as long as possible (to allow time for the air to get behind the mucus). If resistance was too high to maintain the ball at the target level (expiratory flow was too low), it was lowered. Conversely, if the duration of expiration was too short, resistance was increased. In addition, the visual feedback (ascending balls) increased motivation. The physiotherapist stood behind the subject and pressed the sternum to help sustain expiration. Huff-coughs were performed at various lung volumes above residual volume via the chosen resistance until the final cough when mucus was cleared. The breathing exercise was repeated until subjects felt a need to cough, and then 2–3 forced huffs were performed via the Tri-Gym while removing the resistance. If the 3 balls in the Tri-Gym were elevated to maximum during the huff, the huff was considered successful. Cough was spontaneous, and then several forced huffs were performed to remove the mucus. The entire procedure was repeated several times for 20–30 min/session.
The autogenic drainage technique is well established.17 It includes repetitive inspiratory and expiratory maneuvers at various tidal breathing magnitudes with a pause of at least 3 s between inhalations and exhalations. Breathing out is performed in a sighing manner. The pause allows time for the air to get behind the mucus to evacuate it by a subsequent huff. One cycle of 3 phases typically lasts 1–4 min and is repeated until the preset autogenic drainage treatment time of 30 min has elapsed.
Forced expiratory flow-volume maneuvers were performed with the KoKo spirometer (nSpire Health, Longmont, Colorado) according to American Thoracic Society/European Respiratory Society standards.16 An experienced pulmonary technician instructed subjects how to perform the flow-volume maneuver. Emphasis was placed on full inspiration before expiration, without breath-holding, and on forced expiration to residual volume according to recommendations.
Lung Function Data Analysis
The best curves (FVC + FEV1) before and after the breathing therapies were analyzed. The following lung function indexes were analyzed: FVC, FEV1, peak expiratory flow (PEF), and the forced expiratory flow during the middle half of the FVC maneuver (FEF25-75%). The Student paired t test was used (1) to find significant differences before and after breathing exercises determined by the percentage change from baseline for each lung function index and (2) to test the effect of each method on the same subject. A change of <10% for FVC, FEV1, or PEF and a change of ≤20% for FEF25-75% were considered significant. The effects of the breathing exercises on lung function was correlated with sex, age, and pulmonary disease severity. P < .05 was considered significant.
Results
Forty subjects with CF (23 males) were enrolled in the study and performed the resistive-breathing incentive spirometer technique. No adverse events were provoked by breathing through the spirometer; however, 8 subjects complained of transient exhaustion, which disappeared a few minutes after completion of physiotherapy. Eighty-five percent of the subjects reported that they would use the device at home. Thirty-two of 40 subjects also performed the autogenic drainage technique on a different day. The anthropometric data and basic lung function for all subjects are presented in Table 1. There was no significant difference between the groups regarding anthropometric data or baseline lung function.
Anthropometric Data and Basic Lung Function of Subjects
The short-term effect of the therapy method on lung function (% change from baseline) with regard to each of the spirometry parameters is presented in Table 2. Use of the resistive-breathing incentive spirometer resulted in a significant increase in FVC, FEV1, and PEF (P < .001, P < .001, and P = .009, respectively). The FVC and FEV1 of 26 of 40 subjects improved by 5–42%. Mid-flows improved by >20% in 9 (23%) subjects. The use of the autogenic drainage technique did not lead to a significant increase in either of these parameters.
Effect of Therapy Method on Lung Function
The number of subjects (% of population) with improvements in FVC, FEV1, or PEF of ≥10% or FEF25-75% of ≥20% above baseline is presented in Table 3 for both methods. The number of subjects with improvements in FVC, FEV1, and PEF was significantly greater when exercising with the resistive-breathing incentive spirometer. We also found that the spirometer may improve FVC by >10% above baseline at any FEV1 level.
Number of Subjects and Proportion of Population Who Showed Improvement in Lung Function
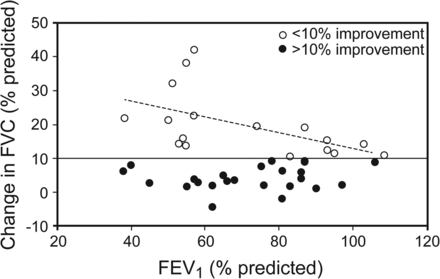
The correlation between baseline percent-of-predicted FVC and improvements after resistive-breathing incentive spirometer therapy is presented in Figure 2. Subjects with an FEV1 of 40–60% of predicted had the greatest improvement in FVC. Improvements demonstrated a negative correlation with baseline percent-of-predicted FVC once improvement was above 10% (r2 = 0.28). Results were similar for FEV1 and PEF (data not shown). FVC improved significantly more in females than males (13.9 ± 10.6 vs 8.2 ± 9.5, P = .042). Improvements in other indexes concerning sex were similar.
Correlation between baseline percent-of-predicted FVC and improvements in percent-of-predicted FEV1 after resistive breathing via a handheld spirometer.
Discussion
In this study, we investigated the short-term effect of expiratory resistive breathing via an incentive spirometer on lung function in a large group of subjects with CF. Our main findings show that use of the resistive-breathing incentive spirometer significantly elevated lung function and especially FVC values in one third of our subjects when measured within 20–30 min after the therapy session, especially when FVC was 40–60% of predicted. We found that the resistive-breathing incentive spirometer technique was well accepted with no adverse events, and the majority of subjects reported that they would use the device at home.
The immediate effect of respiratory physiotherapy is the movement of mucus toward central airways. This may cause a transient obstruction that may negatively alter lung function. It is therefore important to establish the peak-effect time for measuring post-treatment lung function. We measured lung function 20–30 min after resistive-breathing incentive spirometer therapy. This duration is in agreement with the best time for post-therapy measurements in adults with CF as demonstrated by FEV1.18
Our most interesting finding may be the greater improvement in FVC compared with FEV1. The ability to improve FVC was greater in subjects with a baseline FEV1 of 40–60% of predicted. Our findings may be explained by the fact that these subjects may still have parenchymal reserves and thus possess the ability to improve their lung function in moderate lung disease. Because FEF25-75% is FVC- dependent, changes in FEF25-75% may be concealed. Changes in FEV1 may also be concealed if collapse occurs during forced expiration. In that respect, we hypothesize that patients with CF and airway collapse may show improvement in lung function if they perform spirometry against an expiratory resistance such as that used in the resistive-breathing incentive spirometer physiotherapy session. We found that subjects with very low percent-of-predicted FEV1 did not improve as much as subjects with an FEV1 of 40–60% of predicted, who showed improvements of >10%. Our findings were similar to those presented by Placidi et al,20 who studied short-term changes in FEV1 and FEF25-75% after positive airway pressure therapy in 17 subjects with CF and severe airway obstruction (FEV1 < 40% predicted). They did not find any significant effect of this technique on lung function. It should be noted that 25% of our population with severely reduced FEV1 decreased their FEF25-75% by >20% after our resistive-breathing incentive spirometer therapy. We suggest that these findings may indicate the movement of secretions toward central airways and even blocked flow in the small airways and therefore may be expressed as a reduction in FEF25-75%. This suggestion is supported by Chatham et al,22 who found that resistive breathing led to increased expectoration of sputum from the periphery to central airways. In our study, the incidence of females improving their lung function was higher compared with males. The incidence of severe obstruction was also greater in females than in males. This finding could partially explain the greater incidence of significant post-treatment improvements in females. In our study, the autogenic drainage technique did not increase the short-term measurements of lung function, as was found previously for the long-term effect.17
Theoretically, the resistive-breathing incentive spirometer may have several advantages compared with other handheld devices. It is well established that performing expiration via resistance allows air to get behind the mucus, which is essential for mucus clearance to occur.21–23 In our study, we chose the expiratory resistance applied at the mouth that was able to initiate the longest expiration with the highest flow. In return, this chosen resistance may have resulted in a positive pressure sufficient to have been transmitted to the airways, thus holding the airways open during expiration in an optimally controlled fashion. The increased airway pressure during expiration (PEP) is thought to be useful in pushing air through collateral pathways into distal lung units beyond the retained secretions, along with preventing early airway closure, prolonging expiration, and promoting movement of secretions toward central airways.14
All handheld devices employ resistive-breathing techniques.10 The PEP technique suggests that the target resistance should be between 10 and 20 cm H2O during mid-expiration measured by a manometer. With the Flutter device (Axcan Scandipharm, Birmingham, Alabama), the PEP and cyclic oscillation (airway vibration) are produced by lifting a gravity-dependent ball and sustaining it in the air. The Acapella device (Smiths Medical, Watford, United Kingdom) comes in 3 models: low flow (<15 L/min), high flow (>15 L/min), and a dial that sets expiratory resistance. Indeed, these devices were proven to be effective in subjects with mild-to-moderate airway obstruction.22 Still, improvements in lung function were not found. It has been demonstrated that the very high and turbulent flow created by using the Flutter or Acapella instrument may cause early closure of the peripheral airways,22 which may in turn hamper immediate improvements in lung function. Moreover, in exhausted CF patients with severe lung disease, the ability to initiate and build a high flow behind the secretions may be impaired. We suggest that the main advantage of the resistive-breathing incentive spirometer is the ability to adjust resistance through the use of 1–8-mm holes, allowing the operator to choose the desired expiratory resistance. This feature may provide greater opportunities for younger children to use a resistive-breathing incentive spirometer.
Coughing is the body's way of removing mucus from the lungs. However, coughing is not always sufficient to clear the mucus, as hard coughing may lead to airway collapse. The huff-cough is a gentle cough that speeds air flow while keeping the throat open. Indeed, the huff-cough clears airways as effectively as a voluntary cough.3 However, the potential for substantial airway collapse, especially in the smaller bronchioles, still exists, especially because of cartilage damage in diseases such as CF and chronic bronchitis. We suggest that both the visual feedback provided by the ascension of the colored balls and the controlled PEP created by the adequate resistance enable the initiation of huff-coughs, a less injurious, semiforced expiration maneuver at low lung volumes. Furthermore, effective huffs at low lung volumes may improve mobilization of secretions even in decimated lung airways. Indeed, 25% of subjects with severely reduced FEV1 decreased their FEF25-75% by >20% after using our method. This suggestion is supported by Chatham et al,22 who found that resistive breathing led to increased expectoration of sputum from the periphery to central airways.
Finally, enhancing motivation is a great challenge in the treatment of patients with CF.21 The results of our study demonstrate that both immediate (within <30 min) and significant (slower) improvements in pulmonary function testing may greatly increase adherence. Most of our subjects said that they will continue to use this method for airway clearance. In a survey among adults,22 >50% of subjects believed that chest physiotherapy was not effective because they did not see an immediate improvement in lung function. A full chest physiotherapy session takes a minimum of 45 min to 1 h.23 Such a long duration decreases adherence to treatment and increases opposition by older patients and especially by younger patients.24–26
Study Limitations
The final resistance level that each subject used with the Tri-Gym was not documented. Each subject chose the resistance level that provided an appropriate flow. Furthermore, we could not establish the reasons why subjects preferred a specific effort. We assume that the resistance per individual may change with the severity of the disease and with the amount/thickness/viscosity of the secretions. The actual PEP needed to lift a single ball to a sustained target of 600 mL/s (or any other combination) is not provided in the manual for the device, and we do not know of any study in the literature concerning the expiratory pressure needed for these tasks when using the resistive-breathing incentive spirometer. However, the Tri-Gym is a well-known device used around the world for airway clearance therapy after surgery, so it is presumed to fit the standards.27
We did not measure the amount and weight of sputum obtained after resistive-breathing incentive spirometer therapy or compare the amount of sputum induced by this technique versus other techniques. However, the significant increases in FVC, FEV1, and PEF provide objective evidence of improvements that occur after breathing through the modified resistive-breathing incentive spirometer method in subjects with CF. Furthermore, it is important to follow up on the long-term effect of this procedure on lung function deterioration. Such follow-up was beyond the scope of this study.
Conclusions
The incentive volumetric spirometer combined with variable resistance, expiration, and huffs with variable volume offers a unique but simple, friendly, and self-administered method of airway clearance in subjects with CF. We believe that the resistive-breathing incentive spirometer technique has 3 advantages over former handheld devices that enable short-term improvements in lung function. These include approximation of flow passing through an adequate choice of resistance, immediate visual feed-back of ascending colored balls, and the capacity to allow huffs at low lung volumes above the residual volume. The technique has been shown to improve FVC and FEV1, with PEF improving immediately after treatment. Further studies with a larger group of subjects are needed to confirm our findings.
Footnotes
- Correspondence: Daphna Vilozni PhD, Pediatric Pulmonary Unit, The Edmond and Lily Safra Children's Hospital, Sheba Medical Center, Ramat-Gan, Israel, affiliated with the Sackler Medical School, Tel Aviv University, Tel Aviv 52621, Israel. E-mail: daphna.vilozni{at}sheba.health.gov.il.
The study was supported by the J Baum Foundation of the Israel Lung Association (Tel Aviv, Israel).
- Copyright © 2015 by Daedalus Enterprises