Abstract
BACKGROUND: Late inspiratory rise in airway pressure (LIRAP, Paw/ΔT) caused by inspiratory muscle relaxation or expiratory muscle contraction is frequently seen during pressure support ventilation (PSV), although the modulating factors are unknown.
METHODS: We investigated the effects of respiratory mechanics (normal, obstructive, restrictive, or mixed), inspiratory effort (−2, −8, or −15 cm H2O), flow cycle criteria (5–40% peak inspiratory flow), and duration of inspiratory muscle relaxation (0.18–0.3 s) on LIRAP during PSV using a lung simulator and 4 types of ventilators.
RESULTS: LIRAP occurred with all lung models when inspiratory effort was medium to high and duration of inspiratory muscle relaxation was short. The normal lung model was associated with the fastest LIRAP, whereas the obstructive lung model was associated with the slowest. Unless lung mechanics were normal or mixed, LIRAP was unlikely to occur when inspiratory effort was low. Different ventilators were also associated with differences in LIRAP speed. Except for within the restrictive lung model, changes in flow cycle level did not abolish LIRAP if inspiratory effort was medium to high. Increased duration of inspiratory relaxation also led to the elimination of LIRAP. Simulation of expiratory muscle contraction revealed that LIRAP occurred only when expiratory muscle contraction occurred sometime after the beginning of inspiration.
CONCLUSIONS: Our simulation study reveals that both respiratory resistance and compliance may affect LIRAP. Except for under restrictive lung conditions, LIRAP is unlikely to be abolished by simply lowering flow cycle criteria when inspiratory effort is strong and relaxation time is rapid. LIRAP may be caused by expiratory muscle contraction when it occurs during inspiration.
Introduction
Pressure support ventilation (PSV) is frequently used in ventilated patients and is considered a spontaneous mode of ventilation because there is no set respiratory rate and the delivered rate and tidal volume can vary.1,2 A low level of pressure support can be used for patients undergoing a weaning trial, whereas a high level of pressure support can provide additional respiratory muscle unloading.1 The use of PSV is sometimes associated with patient-ventilator asynchrony (eg, trigger asynchrony or double triggering).3,4 To facilitate patient-ventilator synchrony and markedly reduce the asynchrony index, however, a number of variables can be adjusted in pressure support mode, including inspiratory rise time, pressure support level, and flow cycle criteria (ie, expiratory cycle criteria).4
In addition to asynchronies related to inspiratory efforts, expiratory asynchrony occurs when a mechanical breath precedes or follows the end of neural inspiration.5 Premature termination of mechanical ventilation is usually accompanied by distortion of typical flow and airway pressure early during expiration.6 In the case of delayed cycle, an abrupt increase in late inspiratory airway pressure can be observed.6 In an investigation of 15 subjects under PSV with a fixed rise time and flow cycle threshold, the rapidity of diaphragmatic relaxation was found to be the main determinant of airway pressure rise speed in subjects with both restrictive and obstructive lung diseases.7 As delayed cycle beyond the end of inspiratory effort is common in PSV,8 an investigation of possible contributing factors to late inspiratory airway pressure rise with PSV may increase our understanding of expiratory asynchrony. Either relaxation of inspiratory muscles or contraction of expiratory muscles may lead to expiratory asynchrony, but different pathophysiology may lead to different modes of ventilator accommodation.8–10 Therefore, it is of clinical interest to further distinguish between inspiratory muscle relaxation and expiratory muscle contraction as a cause of late inspiratory airway pressure rise. The aim of this study was to determine the factors related to airway pressure rise near the end of inflation during PSV using a simulation lung model. As flow output changes, ventilators rely on the proportional-integral-derivative controllers, which vary according to the difference between current pressure and target pressure and are likely to be different among different ventilator manufacturers. Therefore, we hypothesize different presentations in late inspiratory rise in airway pressure (LIRAP) between different ventilators.8 Additionally, LIRAP is expected when respiratory resistance is low8; however, we hypothesize that respiratory compliance also contributes to LIRAP because of additional cycle criteria in PSV.
QUICK LOOK
Current knowledge
A rise in end-inspiratory pressure during pressure support ventilation (PSV) is associated with inspiratory muscle relaxation and delayed servo control of flow as well as expiratory contraction possibly due to neuromechanical mismatch. This is a common finding during PSV, the etiology of which is difficult to determine at the bedside.
What this paper contributes to our knowledge
In a lung model, simulation of both resistance and compliance impacted the late rise in airway pressure during PSV. Shortening inspiratory time by increasing the flow cycling criteria was successful only in reducing the rise in airway pressure in restrictive lung models when effort was strong and relaxation time was rapid. Expiratory muscle contraction during inspiration creates a late rise in airway pressure based on timing.
Methods
This simulation study was performed using the ASL 5000 computerized lung simulator (IngMar Medical, Pittsburg, Pennsylvania) and a single-compartment, active, linear lung model. Four combinations of resistance and compliance were programmed into the model11: normal (resistance of 5 cm H2O/L/s and compliance of 100 mL/cm H2O), obstructive (resistance of 20 cm H2O/L/s and compliance of 100 mL/cm H2O), mixed (resistance of 10 cm H2O/L/s and compliance of 50 mL/cm H2O), and restrictive (resistance of 5 cm H2O/L/s and compliance of 25 mL/cm H2O). Four types of ventilators were used: PB840 (Covidien, Mansfield, Massachusetts), Maquet Servo-i (Maquet Getinge Group, Rastatt, Germany), Dräger XL (Drägerwerk AG, Lübeck, Germany), and Carestation (GE Healthcare, Madison, Wisconsin).
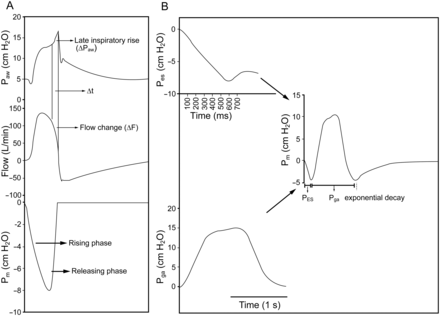
Two study arms were conducted. First, we assessed the influence of inspiratory muscle relaxation using various combinations of lung models and ventilator settings. Next, we inserted expiratory muscle contraction simulations at various time points during the inspiratory phase. A scheme describing the current protocol is shown in Figure 1.
Schematic diagram for this study. Pmax = maximum inspiratory pressure; ETS = expiratory trigger sensitivity, namely flow cycle level.
Effects of Inspiratory Muscle Relaxation
For each of the 4 types of simulated respiratory mechanics, 3 inspiratory efforts (−2, −8, and −15 cm H2O), 2 pressure support levels (5 and 10 cm H2O), and 3 expiratory cycle criteria levels (5%, 20%, and 40% peak inspiratory flow) were used. For the inspiratory phase, the duration of the rising phase was fixed at 0.6 s, and 2 releasing durations (0.18 or 0.3 s; inspiratory relaxation) were used. The PEEP was 5 cm H2O, and breathing frequency was 10 breaths/min. A minimal flow trigger level was adopted without autotriggering. For each setting, 7 breaths were programmed into the ASL 5000 simulator. Air flow was measured via a pneumotachograph connected to a differential pressure transducer, and airway pressure was measured individually using differential pressure transducers (P/N 113252, Model 1110A, Hans Rudolph, Shawnee, Kansas). All signals were sampled and digitalized at 200 Hz, and data were stored in a data acquisition system (AcqKnowledge, Biopac Systems, Goleta, California).
Effects of Expiratory Muscle Contraction
We utilized a user-defined breath profile to simulate expiratory muscle contraction. The effect of expiratory muscle activation in 3 subjects mechanically ventilated under PSV was simulated by timed abdominal compression during the inspiratory phase of a breathing cycle.12,13 The duration of abdominal compression was 1.8 ± 0.8 s, and the average change in gastric pressure was 10.3 ± 6.7 cm H2O. We thus designed 3 gastric pressure templates (a common gastric pressure waveform from a representative subject) with a fixed duration of 2 s and respective pressures of 5, 10, and 15 cm H2O. An inspiratory pressure profile was also selected from the esophageal pressure tracing of the subject and slightly modified to mimic an inspiration with a rising phase interval fixed at 0.6 s under 2 inspiratory efforts (−8 and −15 cm H2O). The selected gastric pressure profiles representing expiratory muscle contraction were inserted 100–700 ms after the start of inspiration. An illustration of these procedures is shown in Figure 2. For placement of esophageal and gastric balloons to obtain esophageal and gastric pressure in subjects, informed consent was obtained from the subjects' next-of-kin. All procedures were approved by institutional review boards, and written informed consent was obtained from all subjects.
A: Simulation of inspiratory muscle relaxation. B: Simulation of expiratory muscle contraction. ΔPaw = airway pressure difference during late inspiratory rise; ΔT = duration of late inspiratory rise; ΔF = flow change during late inspiratory rise; Pm = inspiratory pressure waveform with rising phase interval fixed at 0.6 s; Pes = esophageal pressure; Pga = gastric pressure. A: For this example of inspiratory muscle relaxation, the releasing phase was 0.18 s. B: For this example of expiratory muscle contraction, the maximum gastric pressure was 15 cm H2O.
Dependent Variables
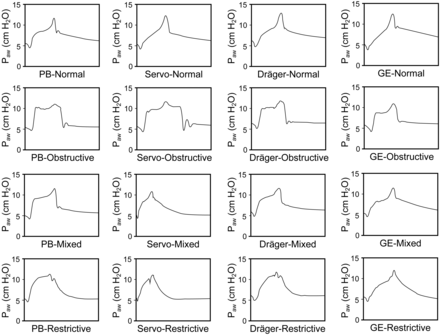
To characterize late inspiratory pressure rise, the slope of airway pressure rise (ΔPaw/ΔT), the corresponding slope of inspiratory flow decrease (Δflow/ΔT), and the duration of end-inspiratory rise time (ΔT) were calculated (see Fig. 2). Current PSV requires a gain factor to determine the flow changes required to achieve target pressure, and a difference between airway pressure and target pressure is frequently observed, especially when changes in alveolar pressure are rapid.8 Therefore, we defined significant end-inspiratory rise as when the slope of the abrupt increase in late inspiratory airway pressure was significantly higher than that of airway pressure before late inspiratory rise. For the restrictive lung model, LIRAP is frequently accompanied by a secondary rise after a notch; only the first LIRAP was taken into account. Representative tracings for the 4 lung models using different ventilators are shown in Figure 3.
Representative tracings for 4 different lung models using 4 different ventilators. Simulation parameters were: medium inspiratory effort of −8 cm H2O, pressure support level of 5 cm H2O, cycling off at 5% peak inspiratory flow, and releasing duration of 0.18 s.
Statistical Analysis
Data are shown as mean ± SD. Comparisons of slopes of late inspiratory rise (ΔPaw/ΔT) and airway plateau were done by linear regression. The Mann-Whitney U test and the Kruskal-Wallis test were used to compare different groups when appropriate. Dunn multiple-comparison tests were performed for pairs of groups. Statistical significance was set at P < .01. Statistical analysis was performed using Prism 5 (GraphPad Software, San Diego, California).
Results
To condense the simulation work, only results obtained at a pressure support level of 5 cm H2O are presented. For results obtained at a pressure support level of 10 cm H2O, see the supplementary materials at http://www.rcjournal.com.
Inspiratory Muscle Relaxation
Effect of Lung Model and Ventilator.
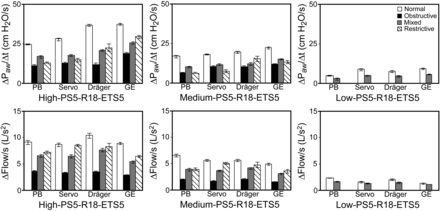
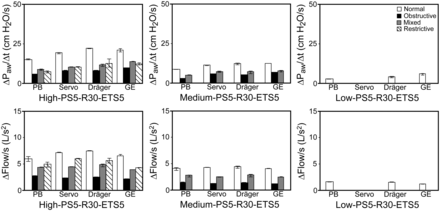
We examined the effects of various inspiratory efforts on ΔPaw/ΔT within 4 different lung models using 4 different ventilators. With low inspiratory effort, we primarily observed significant ΔPaw/ΔT in normal lung models and mixed lung models regardless of the ventilators (Fig. 4). With medium and high inspiratory efforts, we observed significant ΔPaw/ΔT in all lung models with all ventilators when relaxation rate was rapid (0.18 s) and expiratory cycle criteria were low (5% peak inspiratory flow). The highest ΔPaw/ΔT was associated with normal lung models, whereas the lowest ΔPaw/ΔT was associated with obstructive lung models, and the magnitude of ΔPaw/ΔT in restrictive lung models was lower than that in normal lung models despite both lung models having the same resistance. We also observed that Δflow/ΔT was highest in normal lung models and lowest in obstructive lung models with all ventilators (Fig. 4). When we compared the performance of different ventilators, we observed significant differences in ΔPaw/ΔT and Δflow/ΔT with all 4 ventilators. In all lung models, the lowest ΔPaw/ΔT was recorded with the PB840 ventilator and the highest with the Carestation ventilator.
Quantification of late inspiratory airway pressure rise and flow changes in normal, obstructive, mixed, and restrictive lung models using different ventilators under predefined parameters. High inspiratory effort: −15 cm H2O; medium inspiratory effort: −8 cm H2O; low inspiratory effort: −2 cm H2O. PS5 = pressure support level of 5 cm H2O; R18 = relaxation time of 0.18 s; ETS5 = expiratory trigger sensitivity (flow cycle level) of 5% peak inspiratory flow. Significant differences among all lung models with each ventilator were found for ΔPaw/ΔT when inspiratory effort was high or medium. For each lung model, significant differences among all ventilators were found when inspiratory effort was high or medium. For normal and mixed lung models, significant differences were found among all ventilators when inspiratory effort was low. There were also significant differences between normal and mixed lung models with each ventilator when inspiratory effort was low. Similar significance patterns were found for Δflow/ΔT. Data are shown as mean ± SD.
Effect of Expiratory Cycle Criteria.
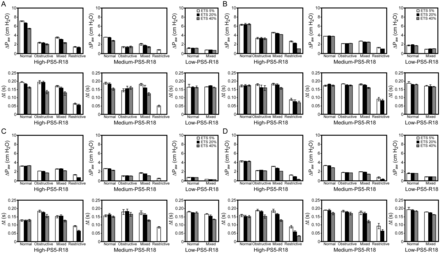
We next examined the effect of different expiratory cycle criteria levels (5%, 20%, or 40%) in the 4 lung models. With high and medium inspiratory efforts with the same relaxation time (0.18 s), ΔPaw and ΔT were affected by different expiratory cycle criteria levels in most lung models with different ventilators (Fig. 5). Lengthening of expiratory cycle criteria completely abolished end-inspiratory rise with either high or medium inspiratory efforts in restrictive lung models. With low inspiratory effort, late inspiratory rise was observed in the normal and mixed lung models at all expiratory cycle criteria levels. However, LIRAP varied widely among the different ventilators.
Effect of flow cycle level for the different ventilators with different lung models under predefined parameters. End-inspiratory rise was abolished by increasing the expiratory cycle criteria level with the restrictive lung model or with low inspiratory effort. High inspiratory effort: −15 cm H2O; medium inspiratory effort: −8 cm H2O; low inspiratory effort: −2 cm H2O. R18 = relaxation time of 0.18 s; ETS = expiratory trigger sensitivity (flow cycle level); PS5 = pressure support level of 5 cm H2O. A: Dräger. Significant differences in ΔPaw and ΔT were found between different expiratory cycle criteria levels in normal, obstructive, and mixed lung models when inspiratory effort was high and in normal and mixed lung model when inspiratory effort was medium. B: GE. Significant differences in ΔPaw and ΔT were found between different expiratory cycle criteria levels in mixed and restrictive lung models when inspiratory effort was high. Significant differences in ΔPaw were found in normal, mixed, and restrictive lung models when inspiratory effort was medium. A significant difference in ΔT was found in mixed lung models when inspiratory effort was medium. C: PB. Significant differences in ΔPaw and ΔT were found between different expiratory cycle criteria levels in obstructive, mixed, and restrictive lung models when inspiratory effort was high. Significant differences in ΔPawwere found in normal, obstructive, and mixed lung models when inspiratory effort was medium. A significant difference in ΔT was found in the mixed lung model when inspiratory effort was medium or low. D: Servo-i. Significant differences in ΔPaw and ΔT were found between different expiratory cycle criteria levels in obstructive, mixed, and restrictive lung models when inspiratory effort was high. Significant differences in ΔPaw and ΔT were found between different expiratory cycle criteria levels in all lung models when inspiratory effort was medium. Significant differences in ΔPaw were found in normal and mixed lung models when inspiratory effort was low.
Effect of Relaxation Time.
We also examined the effect of prolonged inspiratory relaxation (0.3 s) in the 4 lung models at 2 pressure support levels and one expiratory cycle criteria level (5%) with all ventilators (Fig. 6). Overall, compared with short relaxation duration (0.18 s), prolonged inspiratory relaxation significantly reduced the magnitude of ΔPaw/ΔT and Δflow/ΔT (compare Figs. 4 and 6). Moreover, prolonged inspiratory relaxation abolished late inspiratory rise in restrictive lung models, even with medium inspiratory effort. There was no LIRAP in all lung models with the Servo-i ventilator when inspiratory effort was low and inspiratory relaxation was prolonged.
Effect of increased relaxation time (0.3 s) on late inspiratory airway pressure rise and flow changes in different lung models using different ventilators under predefined parameters. Late inspiratory rise could be abolished by increasing relaxation time. High inspiratory effort: −15 cm H2O; medium inspiratory effort: −8 cm H2O; low inspiratory effort: −2 cm H2O. P5 = pressure support level of 5 cm H2O; R30 = relaxation time of 0.3 s; ETS5 = expiratory trigger sensitivity (flow cycle level) of 5%. Significant differences among all lung models with each ventilator were found for ΔPaw/ΔT when inspiratory effort was high. For each lung model, significant differences among all ventilators were found when inspiratory effort was high. For medium inspiratory effort, significant differences within each ventilator were found in normal, obstructive, and mixed lung models. Significant differences were found in the PB, Dräger, and GE ventilators when the inspiratory effort was low. Similar significance patterns were found for Δflow/ΔT. Data are shown as mean ± SD.
Expiratory Muscle Contraction
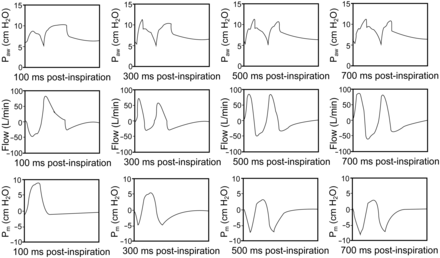
Simulations of expiratory muscle contraction were performed with a user-defined breath. We found that simulation of timed abdominal compression during the inspiratory phase resulted in a late inspiratory rise (∼500 ms after inspiration). This pattern was similar with the simulated expiratory muscle contraction from 5 to 15 cm H2O. Although appropriately timed expiratory muscle compression during the inspiratory phase may lead to a similar late inspiratory rise as that observed for inspiratory muscle relaxation, the associated relaxation of expiratory muscles may trigger another breath (Fig. 7).
Effect of expiratory muscle contraction (Pm) at various time points during the inspiratory phase on occurrence of late inspiratory rise. Time points of expiratory muscle contraction are shown. A late inspiratory rise was observed after a small plateau at 500-ms and 700-ms post-inspiration. Other simulation parameters: pressure support level of 5 cm H2O and cycle at 5% peak inspiratory flow.
Discussion
This simulation study yielded several interesting findings. First, late inspiratory airway pressure rise can occur in all types of lung models if inspiratory effort is medium to high and rate of relaxation is rapid. If inspiratory effort is low, however, an abrupt increase in airway pressure is unlikely to occur unless respiratory mechanics are normal or mixed. Second, a high flow cycle level is unlikely to abolish late inspiratory rise when inspiratory effort is high. Third, despite similar patterns of inspiratory relaxation among different ventilators, there are also sizeable differences in LIRAP among ventilators. Fourth, expiratory muscle contraction may cause late inspiratory rise but only when it occurs sometime after inspiration.
According to a study of 15 subjects carried out by Prinianakis et al,7 inspiratory muscle relaxation accounts for late inspiratory rise during PSV with fixed flow cycle criteria (25% peak inspiratory flow) and 2 types of ventilators (Dräger Evita 2 and PB840). The range of ΔPaw/ΔT and Δflow/ΔT for ventilated subjects using the PB840 ventilator was similar to that observed with medium inspiratory effort in our simulation. Furthermore, in the study by Prinianakis et al,7 the average transdiaphragmatic pressure was 10.6 cm H2O, and the average rate of drop in transdiaphragmatic pressure was 46.51 cmH2O/s, similar to our simulated rate of inspiratory relaxation (44.44 cm H2O/s) with medium inspiratory effort (−8 cm H2O). Therefore, the results of our simulation study appear reliable. As resistance, compliance, and expiratory cycle criteria could be varied, our simulation approach provides a way to examine the isolated effects of these parameters.
As predicted by Younes,8 late inspiratory rise in airway pressure is likely to occur with low resistance. Our simulation revealed that ΔPaw/ΔT was highest in normal lung models, in which respiratory resistance is lowest, whereas ΔPaw/ΔT was lowest in obstructive lung models. Also, different ventilators produce different LIRAP, implicating differences in ventilator flow output in response to an error signal, namely the proportional-integral-derivative controllers that vary among manufacturers. In addition to respiratory resistance, our simulation showed that respiratory compliance may also play a role in LIRAP, as significant ΔPaw/ΔT was rarely observed in restrictive lung models when inspiratory effort was low. One possible explanation is that decreased compliance causes a proportional increase in elastic (alveolar) pressure during the pressure-rising phase of PSV, thereby reducing the gradient between airway and alveolar pressure and resulting in short or no LIRAP.
Cycle criteria in PSV affect the work of breathing in patients recovering from acute lung injury or COPD with exacerbation.14,15 Cycle criteria are also expected to influence inspiratory duration and may possibly decrease LIRAP.8,14,15 Our simulation study revealed that changes in cycle criteria could affect the duration and magnitude of LIRAP in various lung models but could abolish LIRAP only in restrictive lung model when inspiratory effort is medium to high. lung model only. These findings suggest that late inspiratory rise with strong inspiratory effort in mechanically ventilated patients is unlikely to be terminated by simply lowering the cycle level unless patients show restrictive lung mechanics. An explanation for this finding is that the additional cycle criteria (usually upper pressure limit or excessive inspiratory time), which may be activated before flow cycle criteria, were present in all of our tested ventilators.
The speed of relaxation may also play a role in excessive overshoot during PSV. Increased relaxation time abolished LIRAP in a restrictive lung model and significantly decreased ΔPaw/ΔT in all lung models. This may be because more time was available for responding to error signals; therefore, late inspiratory pressure is more likely to be flat.
Expiratory muscle contraction is frequently confused with inspiratory muscle relaxation as a cause of excessive late inspiratory rise.10 Our simulation study suggests that the timing of expiratory muscle contraction is important for the appearance of late inspiratory rise. Expiratory muscle contraction occurring too early during the inspiratory phase will not lead to excessive overshoot because an inspiratory plateau will not appear. Therefore, it is reasonable to speculate that expiratory muscle contraction may lead to an inconsistent end-inspiratory rise8,10 and possibly double triggering, which is different from that observed for inspiratory muscle relaxation.
Some limitations of this simulation study need to be addressed. First, many possible clinical scenarios are beyond the range of parameters examined in our multifaceted simulation. For example, multiple combinations of rising and releasing phases may occur during inspiratory effort.16–18 Second, variation in breathing frequency, ventilator triggering delay, or the occurrence of intrinsic PEEP may provoke patient-ventilator asynchrony, which may generate unexpected airway pressure waveforms, including LIRAP.6 Third, 4 types of ICU ventilators equipped with user-selectable expiratory cycle criteria were used. Additional studies are needed to assess LIRAP during PSV in ventilators with fixed or automated expiratory cycle criteria.5 Despite these limitations, our simulation study implies that a high LIRAP is most likely to be associated with normal respiratory mechanics and strong inspiratory effort. It is the patient factor that dominates this phenomenon, and adjustment of flow cycle criteria may not be effective in its abolishment.
Conclusions
In summary, our study of late inspiratory rise using simulated lung models revealed that both respiratory resistance and compliance may affect LIRAP. Although the duration of LIRAP is affected by cycle criteria, late inspiratory rise is unlikely to be abolished by simply lowering flow cycle criteria when inspiratory effort is strong and relaxation time is rapid except under restrictive lung conditions. Thus, a high LIRAP during PSV is an indication to a clinician that the existence of high inspiratory effort from the patient first should be considered, and management should be directed toward the underlying cause. Also, expiratory muscle contraction may lead to LIRAP when it occurs at specific times during inspiration.
Footnotes
- Correspondence: Chang-Wen Chen MD MSc, National Cheng Kung University Hospital, 138 Sheng-Li Road, Tainan 704, Taiwan. E-mail: cwchen{at}mail.ncku.edu.tw.
Supplementary material related to this paper is available at http://www.rcjournal.com.
The study was supported by grant NCKUH-10202047 from the National Cheng Kung University Hospital, Tainan City, Taiwan. The authors have disclosed no conflicts of interest.
- Copyright © 2015 by Daedalus Enterprises