Abstract
BACKGROUND: Oxidative stress and inflammatory responses are thought to be involved in the pathogenesis of ARDS, which is one of the most serious complications of orthotopic liver transplantation (OLT). The collection of exhaled breath condensate (EBC) is a noninvasive method for obtaining clinical samples from the lungs. However, the changes of mediators of inflammation and oxidative stress in EBC remain unclear. Therefore, the aim of this study was to investigate the changes of mediators in EBC from OLT subjects and the relations between these mediators and ARDS.
METHODS: The levels of mediators of oxidative stress (superoxide dismutase [SOD], malondialdehyde [MDA], H2O2, NO, and 8-iso-prostaglandin F2α) and of inflammatory factors (tumor necrosis factor-α[TNF-α], interleukin [IL]-8, and IL-10) were measured in EBC and serum samples collected from 28 subjects before OLT surgery and at 2 and 4 h after the anhepatic phase. The diagnostic value for ARDS until the 3 days following transplantation was evaluated.
RESULTS: Eighteen subjects developed ARDS after OLT. The concentrations of TNF-α, IL-8, MDA, NO, H2O2, and 8-iso-prostaglandin F2α were much higher in the ARDS group than in the control group, whereas the levels of IL-10 and SOD were lower in the ARDS group than in the control group. The serum levels of mediators of oxidative stress or inflammation were closely related to EBC levels. Receiver operating characteristic analysis showed that areas under the curves for MDA, NO, H2O2, 8-iso-prostaglandin F2α, TNF-α, IL-8, SOD, and IL-10 were 0.88, 0.88, 0.78, 0.84, 0.84, 0.94, 0.81, and 0.84 at 2 h after graft reperfusion and 0.98, 0.88, 0.92, 0.79, 0.95, 0.83, 0.88, and 0.97 at 4 h after graft reperfusion.
CONCLUSIONS: EBC analysis is a noninvasive method for detecting mediators of inflammation and oxidative stress from the lungs. This method could be used to predict the higher incidence of ARDS induced by OLT.
Introduction
ARDS is a common complication of orthotopic liver transplantation (OLT) and has high morbidity and mortality rates.1 However, the diagnosis of ARDS is indirect and based on arterial blood gas measurements, bronchial lavage fluid analysis, and chest x-ray findings, which are considered to lag behind the clinical picture, such as increasing respiratory distress and tachycardia.2 A new, noninvasive method for the early prediction of ARDS induced by OLT is urgently needed.
Much pulmonary research over the past several decades has focused on noninvasive methods for detecting biochemical markers in the airway. One such method involves the collection of exhaled breath condensate (EBC).3 EBC analysis is a relatively new technique for exploring the role of inflammation in respiratory diseases that is convenient and also avoids lung injury. However, whether the molecules measured in EBC are associated with pulmonary inflammation or oxidative stress remains unclear.4
Although it is known that inflammatory responses and oxidative stress are primarily responsible for ARDS, measurements of mediators of inflammation and oxidative stress during liver transplantation have been neglected.5,6 Thus, we analyzed changes in the levels of these mediators in EBC and in serum from OLT subjects and investigated their relations with postoperative ARDS. We hypothesized that increases in the levels of inflammatory and oxidative mediators in EBC would predict an increased risk of postoperative ARDS after OLT.
QUICK LOOK
Current knowledge
Oxidative stress and inflammatory response are implicated in the pathogenesis of ARDS. Exhaled breath condensate may be a noninvasive method for obtaining clinical samples from the lungs. To date, the presence and alterations of inflammatory mediators and markers of oxidative stress in exhaled breath condensate have not provided clinically relevant information.
What this paper contributes to our knowledge
In a small group of subjects developing ARDS following liver transplantation, analyses of exhaled breath condensate generated a noninvasive index to predict the risk of postoperative ARDS. The utility of these data in this and other populations to predict the development of ARDS requires further study.
Methods
Subjects
This study was approved by the Research Committee of the Third Affiliated Hospital of Sun Yat-Sen University, and informed consent was obtained from the legal caretaker of each participant (reference CHICTR-PNRC-0800043). Twenty-eight adult subjects with end-stage cirrhosis (18 males and 10 females) with American Society of Anesthesiologists statuses of II–IV underwent modified piggyback liver transplantations. Among them, 14 had cirrhosis due to hepatitis B, 12 had cirrhosis with small liver cancer tumors, and 2 had primary biliary cirrhosis. Twelve subjects were categorized as Child's grade B, 10 subjects as grade C, and 6 as grade A. Two subjects had hepatic encephalopathy grade I, and one subject had hepatic encephalopathy grade II.
Anesthesia and Intra-operative Management
Subjects were placed under general anesthesia. Anesthesia was induced with propofol (1–2 mg/kg) and fentanyl (5 μg/kg) administered intravenously, and neuromuscular blockade was accomplished with cisatracurium (0.1 mg/kg, administered intravenously). Subjects were mechanically ventilated in a volume-controlled mode with an anesthesia ventilator (Datex-Ohmeda, Madison, Wisconsin). The ventilator settings consisted of a tidal volume (VT) of 8–10 mL/kg, a breathing frequency of 10–14 breaths/min, and an inspiratory/expiratory ratio of 1.5; the end-tidal carbon dioxide CO2 tension was maintained between 30 and 35 mm Hg. The PEEP was set at 0 cm H2O. The VT was not manipulated until PaO2 was below 300 mm Hg and SpO2 was lower than 98%. We adjusted the respiratory parameters, such as increasing of PEEP, decreasing the tidal volume, and increasing the oxygen concentration. General anesthesia was maintained with 1.0–3.5% sevoflurane in a mixture of 60% oxygen-air. After the induction of anesthesia, direct arterial pressure was monitored by the left radial artery, and a pulmonary artery catheter was then inserted to measure cardiac output. After OLT, subjects were transferred to the ICU, and mechanical ventilation was continued until successful extubation was achieved.
Definitions of Each Perioperative Phase
The anhepatic phase is the period from vascular clamping to reperfusion of the portal vein and the inferior vena cava, and the neohepatic phase is the period from reperfusion of the donor liver to the end of the operation.
Monitoring of Respiratory Mechanics and Oxygenation
Respiratory parameters, including the VT, dynamic compliance, peak inspiratory pressure (PIP), breathing frequency, mean airway pressure, and plateau pressure, were measured. Some other oxygenation parameters, such as respiratory index (RI), PaO2/FIO2 ratio (P/F), and P(A-a)O2 were calculated according to the following formulas7: P/F = PaO2/FIO2, P(A-a)O2 = PAO2 − PaO2, PAO2 = PIO2 − (1.25 × PaCO2), PIO2 = (760 − 47) × FIO2, and RI = P(A-a)O2/PaO2.
Collection of Exhaled Breath Condensate
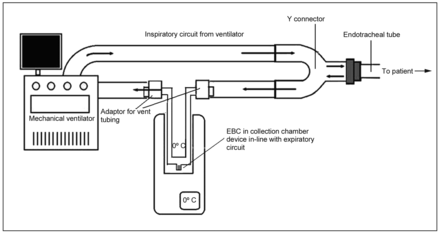
The EBC collection device was constructed using a portable glass tube in line with the expiratory limb of the ventilator circuit (Fig. 1). The glass tube was cooled by being surrounded with a mixture of ice and water to generate temperatures reaching 0°C. Once the collection was finished, the glass tube was disconnected, and the sample was stored immediately at −80°C. The EBC samples were collected at four time points: T1 (5 min after induction of anesthesia), T2 (2 h after graft reperfusion), T3 (4 h after graft reperfusion), and T4 (24 h after graft reperfusion).
The exhaled breath condensation (EBC) collection device consisted of a portable glass tube in line with the expiratory limb of the ventilator circuit. The glass tube was cooled with a mixture of ice and water surrounding the tube to generate temperatures reaching 0°C.
Blood Sample Collection
Radial artery blood samples (4 mL) were collected at T1, T2, T3, and T4 and divided into 2 aliquots. One aliquot (2 mL) was promptly used for blood gas analysis, and the other (2 mL) was used for measurements of mediators of inflammation and oxidative stress.
Assays for Mediators of Inflammation and Oxidative Stress
All EBC and serum samples were divided into 2 aliquots. One set of EBC and serum aliquots was assayed for TNF-α, IL-8, IL-10, and 8-iso-prostaglandin F2α using a commercially available enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, Minnesota) according to the manufacturer's instructions. The other set of EBC and serum aliquots was assayed for SOD, MDA, NO, and H2O2 using commercially available assays (Jiancheng Bioengineering, Nanjing, China) according to the manufacturer's instructions.
Definitions of Outcome Variables
Postoperative ARDS.
Subjects were considered to have ARDS if they met the 2012 Berlin definition of ARDS definition,8 which required the development of ARDS within 3 d after OLT. ARDS was based on degree of hypoxemia: mild (200 mm Hg < PaO2/FIO2 ≤ 300 mm Hg), moderate (100 mm Hg < PaO2/FIO2 ≤ 200 mm Hg), and severe PaO2/FIO2 ≤ 100 mm Hg). All subjects received the same therapies before experiencing ARDS, and standardized treatment was made for those who developed ARDS.
Postoperative Pulmonary Infection.
Pulmonary infections that developed within 3 d after transplantation were recorded, but preoperative pulmonary infections were not recorded for this study.
Other Complications.
Secondary outcome measures including the amount of bleeding, transfusion volume, time to extubation, in-hospital mortality rate, postoperative stay, and the stay in the ICU were all recorded.
Statistical Analysis
The chi-square test of independence was used to examine categorical variables, and independent sample t tests were used to compare continuous characteristics between 2 groups. Areas under the curve were calculated from standard receiver operating characteristic plots. An area under the curve of 0.5 is no better than that expected by chance, whereas a value of 1.0 signifies a perfect biomarker. Relationships between the inflammatory or oxidative mediators in EBC and those in serum were analyzed using Pearson correlation coefficients. P < .05 was considered statistically significant. SPSS 16.0 (SPSS, Chicago, Illinois) was used to generate all statistical analyses and figures.
Results
General State of Health
All operations were successful. Among the 28 subjects who participated in this study, 18 subjects suffered from ARDS after OLT, including 9 cases with mild ARDS, 5 cases with moderate ARDS, and 4 cases with severe ARDS. No sepsis or gastric aspiration occurred in any of the 28 subjects. As shown in Table 1, no differences were observed between the 2 groups in regard to age, weight, sex, height, preoperative MELD (Model for End-stage Liver Disease) score, Child-Pugh score, ascites, concentrated red blood cells, fresh frozen plasma, platelet, albumin and cryoprecipitate, intra-operative urine output, anhepatic time, liver cold ischemia time, and duration of operation.
Characteristics and Outcomes of OLT Subjects
Perioperative Respiratory Mechanics and Oxygenation Parameters
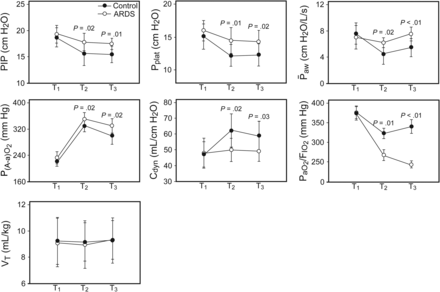
Pulmonary function may be related to respiratory mechanics and oxygenation parameters.9 Thus, in the current study, we measured subjects' perioperative respiratory mechanics and oxygenation parameters, such as PIP, mean airway pressure, P(A-a)O2, P/F, and dynamic compliance. As shown in Figure 2, PIP, mean airway pressure, and P(A-a)O2 were significantly higher in the ARDS group at T2 and T3 compared with the control group (Fig. 2). However, there were no significant differences in VT at T2 and T3 between the ARDS group and the control group (P = .25; 0.18). In addition, P/F and dynamic compliance were significantly lower at T2 and T3 compared with the control group (Fig. 2). These results suggest that there were slight disturbances in respiratory mechanics early on in the ARDS group.
Peak inspiratory pressure (PIP), mean airway pressure (P̄aw), and P(A-a)O2 were significantly higher in the ARDS group than in the control group at T2 (2 h after graft reperfusion) and T3 (4 h after graft reperfusion). However, there was no significant difference in tidal volume between the ARDS and control groups. In addition, PaO2/FIO2 and dynamic compliance (Cdyn) were significantly lower at T2 and T3 compared with the control group. Data are shown as mean ± SD.
Inflammatory Mediators in EBC and Serum
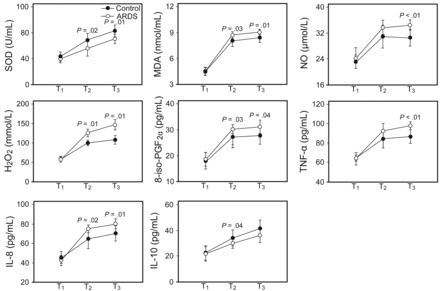
It has been shown that inflammatory mediators play a key role in the pathogenesis of ARDS, but the role of inflammatory mediators in EBC during OLT remains unclear.10 In the present study, we found that the levels of TNF-α and IL-8 in EBC in the ARDS group were significantly higher than in the control group but that the concentrations of IL-10 were much lower in the control group. Similarly, higher TNF-α and IL-8 concentrations and lower IL-10 concentrations were found in serum in the ARDS group compared with the control group (Figs. 3 and 4).
Levels of tumor necrosis factor-α (TNF-α) and interleukin (IL)-8 in exhaled breath condensate (EBC) were significantly higher in the ARDS group compared with the control at T3 (4 h after graft reperfusion), but the concentration of IL-10 was much lower in the ARDS group at T2 (2 h after graft reperfusion) than in the control group. Higher levels of malondialdehyde (MDA), H2O2, and 8-iso-prostaglandin F2α (8-iso-PGF2α) and lower concentrations of superoxide dismutase (SOD) were found in EBC in the ARDS group compared with the control group at T2 and T3. Data are shown as mean ± SD.
Higher tumor necrosis factor-α (TNF-α) and interleukin (IL)-8 concentrations and lower IL-10 concentrations in serum were found in the ARDS group compared with the control group at T2 (2 h after graft reperfusion) and T3 (4 h after graft reperfusion). The levels of malondialdehyde (MDA), NO, H2O2, and 8-iso-prostaglandin F2α (8-iso-PGF2α) in serum were all higher than in the ARDS group at T2 and T3. Data are shown as mean ± SD. SOD = superoxide dismutase.
Mediators of Oxidative Stress in EBC and Serum
In this study, we found higher levels of MDA, NO, H2O2, and 8-iso-prostaglandin F2α in the ARDS group at T2 and T3 in both EBC and serum compared with the control group. However, the concentration of SOD followed the opposite trend at T2 and T3 (Figs. 3 and 4).
Complications and Outcomes
The time to extubation, presence of postoperative pulmonary infection, stay in the ICU, and the postoperative stay were all markedly higher or longer in the ARDS group than in the control group (Table 1). Further analysis indicated that the levels of TNF-α, IL-8, IL-10 MDA, NO, H2O2, and 8-iso-prostaglandin F2α in EBC at T2 (r = 0.49, P = .009; r = 0.59, P = .001; r = 0.53, P = .004; r = 0.44, P = .018; r = 0.49, P = .008; and r = 0.45, P = .017, respectively) and T3 (r = 0.43, P = .022; r = 0.77, P = .004; r = 0.39, P = .04; r = 0.43, P = .023; r = 0.40, P = .037; and r = 0.57, P = .002, respectively) all correlated significantly with the time to extubation.
Six subjects suffered from pulmonary infections in the ARDS group after OLT. No subject in the control group suffered from a pulmonary infection. There was no acute renal failure or cardiac insufficiency after surgery. Two subjects died within the first year after discharge: one died of multiple organ failure, and the other died from a recurrence of hepatoma.
Correlation Analysis
Regression analysis showed the following results: (1) There were no significant correlations between the levels of TNF-α, IL-8, IL-10 MDA, NO, H2O2, SOD, and 8-iso-prostaglandin F2α in EBC and those in serum at T1 (r = 0.16, P = .38; r = 0.19, P = .32; r = 0.30, P = .11; r = 0.15, P = .43; r = 0.05, P = .83; r = 0.30, P = .11; r = 0.03, P = .89; and r = 0.22, P = .26, respectively); (2) the levels of IL-8, IL-10 MDA, H2O2, and 8-iso-prostaglandin F2α in EBC were significantly correlated with those in serum at T2 (r = 0.43, P = .03; r = 0.51, P = .01; r = 0.47, P = .01; r = 0.44, P = .01; and r = 0.49, P = .01, respectively); and (3) the levels of TNF-α, IL-8 MDA, NO, H2O2, and 8-iso-prostaglandin F2α in EBC were significantly correlated with those in serum at T3 (r = 0.67, P < .001; r = 0.47, P = .01; r = 0.71, P < .001; r = 0.59, P < .001; r = 0.61, P < .001; and r = 0.523, P = .005, respectively).
Receiver Operating Characteristic Curve Analyses
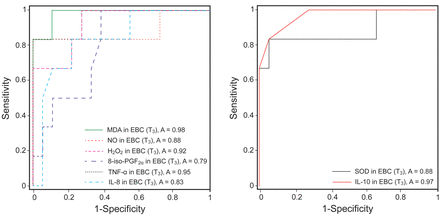
Figure 5 shows the performances of the biomarkers of inflammation and oxidative stress in diagnosing ARDS at 2 h after graft reperfusion. The areas under the curve are: 0.88 for MDA, 0.88 for NO, 0.78 for H2O2, 0.84 for 8-iso-prostaglandin F2α, 0.84 for TNF-α, 0.94 for IL-8, 0.81 for SOD, and 0.84 for IL-10 in EBC.
Receiver operating characteristic curves for biomarker levels in exhaled breath condensate (EBC) at 2 h after graft reperfusion. The areas under the curve are as follows: 0.88 for malondialdehyde (MDA), 0.88 for NO, 0.78 for H2O2, 0.84 for 8-iso-prostaglandin F2α (8-iso-PGF2α), 0.84 for tumor necrosis factor-α (TNF-α), 0.94 for interleukin (IL)-8, 0.81 for superoxide dismutase (SOD), and 0.84 for IL-10, in EBC.
Similarly, Figure 6 shows the performances of the biomarkers of inflammatory and oxidative stress in diagnosing ARDS at 4 h after liver transplantation. The areas under the curve are: 0.98 for MDA, 0.88 for NO, 0.92 for H2O2, 0.79 for 8-iso-prostaglandin F2α, 0.95 for TNF-α, 0.83 for IL-8, 0.88 for SOD, and 0.97 for IL-10 in EBC.
Receiver operating characteristic curves for biomarker levels in exhaled breath condensate (EBC) at 4 h after graft reperfusion. The areas under the curve are as follows: 0.98 for malondialdehyde (MDA), 0.88 for NO, 0.92 for H2O2, 0.79 for 8-iso-prostaglandin F2α (8-iso-PGF2α), 0.95 for tumor necrosis factor-α (TNF-α), 0.83 for interleukin (IL)-8, 0.88 for superoxide dismutase (SOD), and 0.97 for IL-10, in EBC.
Discussion
Over the past several decades, much progress has been made in liver transplantation, but the incidence of postoperative ARDS has not fallen appreciably.11 Thus, it has become increasingly important to develop an ability to predict and diagnose ARDS as early as possible.12 In this study, we found that levels of inflammatory factors (TNF-α and IL-8)5 and mediators of oxidative stress (MDA, H2O2, NO, and 8-iso-prostaglandin F2α)13,14 in both EBC and serum were significantly elevated in subjects with ARDS but that concentrations of IL-10 (an anti-inflammatory cytokine) and SOD (an antioxidant) in EBC and serum both decreased in subjects with ARDS. We also found that all these mediator increases correlate with the incidence of ARDS following OLT and some other poor prognoses. To our knowledge, serum is always affected by fluid infusion or blood loss, and EBC can be obtained directly and noninvasively from the lungs and reflects the true state of the lungs.15 Furthermore, our findings suggest that subjects with higher levels of mediators of inflammation and oxidative stress had a higher risk of developing pulmonary infection postoperatively and staying longer in the ICU, resulting in a poor prognosis and poor recovery.
Currently, ARDS can be diagnosed by blood gas analysis, bronchoalveolar lavage fluid analysis, and chest x-ray findings.16 However, these diagnostic methods either tend to lag behind the clinical picture or are traumatic for patients. Fortunately, noninvasive methods, such as measurements of EBC, have also been used to monitor respiratory disease.17 EBC analysis is a new and noninvasive technology that can detect biochemical markers in the airway and has been reported to be used for the diagnosis of respiratory diseases. To collect EBC samples, multiple custom devices that use a variety of cooling techniques have been used throughout the years.18 Effective collection requires a stable collection device that can provide adequate minute ventilation and a low cooling temperature.19 In the current study, our EBC collecting device consisted of glass chambers connected to the expiratory limb of the ventilator circuit (Fig. 1). Once an EBC sample is collected, it should be transferred promptly to liquid nitrogen and stored at −80°C to prevent rapid degradation. Our EBC collection device was also closed to prevent substances in our EBC samples from evaporating.
The pathogenesis of ARDS is complex and includes activation of pulmonary endothelium and macrophages and upregulation of adhesion molecules and cytokines, all of which result in a massive sequestration of neutrophils within the pulmonary microvasculature.20 These cells release a variety of pro-inflammatory and anti-inflammatory mediators that diffuse into the circulation and other tissues.21 To date, nearly all clinical research on inflammatory mediators in OLT has focused on the presence of these mediators in serum to confirm the existence of lung inflammation.22,23 However, measurements of these inflammatory mediators in OLT lag behind clinical reality. In this study, we found that the trends of these mediators in EBC were closely related to their trends in serum and that measurements of these mediators in EBC directly reflected the state of the lungs during and after liver transplantation. These findings suggest that EBC collection and evaluation are valuable for the diagnosis of ARDS. The causes of postoperative ARDS after liver transplantation include many aspects, such as fluid overload from crystalloid liquid infusion or massive transfusion, hypotension, sepsis, fast tapering of corticosteroids, gastric aspiration, reperfusion syndrome of the newly implanted liver, intestinal ischemia and reperfusion, and transfusion-related acute lung injury.24 Consistent with the findings of some studies,25–27 we believed that not only inflammation but also oxidative stress were involved in the mechanism of ARDS. Our findings also indicated higher levels of mediators of inflammation and oxidative stress in EBC, indirectly showing the key role of inflammation and oxidative stress in ARDS after liver transplantation. It has also been suggested that the severity of ARDS could be reduced by actions on several pathways, such as inhibiting the production of oxygen free radicals (such as H2O2) and nitrate radicals (such as NO) and reducing pulmonary oxidative damage that is accompanied by increased levels of MDA and decreased levels of SOD.26,27 Our results demonstrated that the levels of mediators of oxidative stress detected in EBC followed the same trend as those in serum, which closely correlated with the incidence of ARDS. These results also suggest that pulmonary oxidative stress may have potentially serious consequences and that the levels of mediators of oxidative stress in EBC have the potential to predict the degree of oxidative stress in OLT patients.28
Several limitations of this study should be acknowledged. First, although EBC detection is direct, it is not instantaneous,29 and this delay could affect the accuracy of results obtained from the analysis of EBC. Second, it is notable that serum TNF-α concentration in this study was 10 times higher than that reported in another study.30 We inferred that the reading of TNF-α was influenced by many factors, including the severity of liver disease, manufacturers of detection devices, and laboratory temperature. Interestingly, the value of TNF-α in this study was similar to that of another study,31 which was related to the inflammatory reaction. Third, it seemed that lung compliance improved only in the control group, whereas it remained almost constant in the ARDS group. We learned from another study that removal of the ascites in subjects undergoing liver transplantation tended to improve dynamic lung compliance,32 which was consistent with the control group in our study. However, we found that lung compliance decreased after transplantation in the ARDS group, which might suggest worsening respiratory condition. Fourth, there was no mortality related to ARDS in the current study. However, according to the data from another study,8 the mortality rate was expected to be markedly higher than ours. After detailed analysis of our results, we found that the ARDS subjects were treated very early and effectively. Furthermore, less bleeding, blood transfusion, and urine volume, as well as shorter operation time, might also correlate with the low mortality. Additionally, no mortality might be due to the small sample size, and the mortality may increase if we enlarge the sample size.
Conclusions
We showed that analyses of EBC obtained directly from the lungs could be used to generate a noninvasive index to predict the risk of postoperative ARDS after OLT. However, the new technique of EBC has not yet become a routine method of diagnosis for ARDS, and its practical value needs further study.
Footnotes
- Correspondence: Ziqing Hei PhD, Department of Anesthesiology, Third Affiliated Hospital of Sun Yat-sen University, No. 600 Tianhe Road, Tianhe District, Guangzhou City 510630, People's Republic of China. E-mail: heiziqing{at}sina.com.
Drs Liu and G Luo are co-first authors of this paper.
This study was supported by grant 2011B031800058 from the Scientific and Technological Project Foundation of Guangdong Province (People's Republic of China), grant 82000-3281901 from the 985 Project of China, and grant 2011B0929 from the Braun Scientific Research Fund for Anesthesia.
The authors have declared no conflicts of interest.
- Copyright © 2015 by Daedalus Enterprises