Abstract
BACKGROUND: Impaired spirometric parameters have been reported in patients with stage C heart failure and portend worse outcomes in these patients. The impact of spirometric parameters on outcomes in patients with stage D heart failure listed for heart transplantation is unknown.
METHODS: We collected data on consecutive subjects listed for heart transplantation and examined the association of FEV1, FVC, and FEV1/FVC with (1) death or left ventricular assist device implantation (primary end point) and (2) death, left ventricular assist device implantation, or urgent transplantation (secondary end point). In a secondary analysis, we examined the association of baseline spirometry with post-transplant outcomes.
RESULTS: Among 187 subjects (53 ± 10 y old, 17.1% women, 69.5% white subjects, 28.9% black subjects), there were 19 deaths, 28 left ventricular assist device implantations, and 74 urgent transplantations (primary end point of 25.1%, secondary end point of 64.7%) after a median of 5.5 months (interquartile range of 2.3–15.2). For FEV1, the hazard ratios for the primary and secondary end points were 0.93 (95% CI 0.61–1.41, P = .72) and 0.94 (95% CI 0.72–1.21, P = .62) per L, respectively. The hazard ratios of FVC were 0.90 (95% CI 0.65–1.25, P = .52) and 0.92 (95% CI 0.76–1.13, P = .43) per L, respectively. Impairment patterns (obstructive, restrictive, mixed) were not associated with risk for events. There was no interaction of spirometric parameters with smoking or lung disease for outcomes. Baseline spirometry was not associated with perioperative 30-d mortality (1.4%) and 1-y post-transplant survival (97.1%).
CONCLUSIONS: In contrast to stage C subjects with heart failure, spirometric parameters were not associated with outcomes in this homogeneous stage D heart failure population.
Introduction
Heart failure is a chronic debilitating condition with increasing prevalence and significant morbidity and mortality.1–4 Improved outcomes of acute cardiac conditions, population aging, increasing prevalence of lifestyle-related risk factors, and advances in heart failure therapy have all led to an ever-increasing prevalence of heart failure. Because of these trends, heart failure is currently considered an epidemic and a public health priority in developed countries.5–7 Approximately 5–10% of the heart failure population is estimated to have advanced (stage D) heart failure.8 Mortality in this group remains high, with projected 2-y survival of < 40%.9 Despite advances in both medical and destination left ventricular assist device therapy, heart transplantation remains the optimal intervention for long-term survival and quality of life for patients with stage D heart failure. A 1-y post-transplant survival of 90%, with a 50% survival rate noted at 11 y, has been reported.10
Despite improvements in transplant outcomes, donor heart availability continues to be the main barrier, with annual transplant numbers remaining stagnant at ∼2,000 transplants/y from 2000 to 2011 in the United States.11 With high demand and limited availability, the transplant evaluation process remains tenuous. Patients are evaluated in terms of physical, psychological, and social attributes, with some inter-institutional variation in the process. Among the absolute contraindications for heart transplantation is significant obstructive pulmonary disease,8 underscoring the importance of pulmonary function for transplant outcomes. Thus, spirometry is an inherent part of the evaluation process.8 Both obstructive and restrictive patterns have been seen in subjects with heart failure and no history of tobacco use or prior lung or pleural space disease.12–15 Studies have shown a correlation between severity of pulmonary function abnormalities and severity of heart failure.12,16 Of note, subjects with advanced heart failure were reported to have more severe abnormalities than those with milder presentations.12,17
Impaired pulmonary function has also been proposed as a cardiovascular risk factor18 and has been associated with all-cause and cardiovascular mortality.19,20 Specifically to heart failure, spirometric parameters have been demonstrated to predict incident heart failure21–23 and outcomes of patients with stage C heart failure.17,24–26 However, the association of spirometric parameters with outcomes in patients with advanced (stage D) heart failure listed for heart transplantation is less clear. Patients referred for heart transplantation represent a more homogeneous population in which left ventricular function is severely impaired and congestive symptoms are common.
In this study, we investigated the association of resting spirometric parameters with outcomes (mortality, need for left ventricular assist device implantation, or urgent heart transplantation) in a cohort of subjects listed for heart transplantation. In a secondary analysis, we also examined the association of baseline spirometry with post-transplant survival.
QUICK LOOK
Current knowledge
Heart failure is a chronic debilitating condition with an increasing prevalence and significant morbidity and mortality. Approximately 5–10% of patients with heart failure have advanced (stage D) disease. Mortality remains high, with a projected 2-y survival of < 40%. Heart transplantation remains the optimal intervention for long-term survival and quality of life, with a 1-y post-transplant survival of 90%.
What this paper contributes to our knowledge
Spirometric parameters were not associated with outcomes in the homogeneous group of subjects with heart failure listed for heart transplantation. Severe heart failure led to significant reductions in FEV1 and FVC, suggesting that the majority of patients with advanced heart failure will have impaired spirometric values.
Methods
Subject Population
We collected data on consecutive adults (≥ 18 y) listed for heart transplantation between January 2000 and December 2012 at Emory University. Patients with heart failure secondary to congenital heart disease were excluded. A total of 345 subjects fulfilling these criteria were listed for heart transplantation: 43 (12.5%) were listed as United Network for Organ Sharing (UNOS) status 1A, 153 (44.3%) as status 1B, and 149 (43.2%) as status 2. Among these subjects, 187 (54.2%) had spirometric data available and constituted our main study population for this analysis. The institutional review board approved the study.
Data Collection
Demographics, clinical information, and laboratory and hemodynamic data were abstracted from paper and electronic medical records. We considered the date of initial listing status as the baseline date for the study. Medical history, physical examination, and laboratory data were abstracted from the clinic visits closest to the date of listing. Subjects were regularly followed in the Center for Heart Failure and Transplantation at Emory University. Information on outcomes was collected through medical records from planned and unplanned clinic visits, admissions, surgical procedures, in-hospital progress notes, and discharge summaries. The vital status of subjects censored as alive as of December 2012 was confirmed through the scheduling and communication modules of the Emory electronic health records system. Out-of-hospital deaths are captured in the Emory Clinical Data Warehouse through linkage to the Social Security Death Index. No subject was lost to clinical follow-up.
Definition of Variables
History of smoking and alcohol abuse was considered as present based on information given by the subjects. History of myocardial infarction, coronary artery bypass grafting, and percutaneous coronary intervention was considered as positive when there was information about a previous event, surgery, and intervention written in subjects' notes in the medical history. We defined prevalent coronary artery disease as history of myocardial infarction, coronary artery bypass grafting, or percutaneous coronary intervention. History of hypertension, diabetes, dyslipidemia, chronic lung disease, sleep apnea, CPAP use, depression, atrial and ventricular arrhythmias, and cardiac device implantation (biventricular pacemaker, automatic implantable cardiac defibrillator, or both) was based on documentation of these conditions in subjects' medical records by the time of listing for heart transplantation. We defined lung disease as the presence of COPD, asthma, or sarcoidosis. Atrial arrhythmias included permanent and paroxysmal atrial fibrillation or flutter. Information on medical history was considered valid when it was reported in more than one note in the medical record. Because of the possibility of interaction between smoking, lung disease history, and diabetes mellitus, a condition that has been reported to affect pulmonary function,27 we tested for possible interaction between spirometric variables and smoking, lung disease, and diabetes.
Spirometry
Spirometry was performed with a horizontal dry rolling-seal spirometer (SensorMedics, Yorba Linda, California) with subjects in a sitting position using the acceptability and reproducibility criteria and the selection of maneuvers proposed in the recommendations of the American Thoracic Society.28 In this analysis, we considered absolute and percent-of-predicted FEV1 and FVC and the FEV1/FVC ratio. Spirometric obstructive ventilatory pattern was defined as a combination of FEV1/FVC < 0.70 and FEV1 < 80% of predicted; restrictive ventilatory pattern was defined as a combination of FEV1/FVC ≥ 0.70 and FVC < 80% of predicted; and mixed pattern was defined as a combination of FEV1/FVC < 0.70, FEV1 < 80% of predicted, and FVC < 80% of predicted.29
Outcomes
We collected data on vital status, left ventricular assist device implantation, heart transplantation, and UNOS status at the time of transplantation. The primary end point of interest was the time to death or left ventricular assist device implantation (whichever occurred first). The secondary end point was defined as death or urgent transplantation (UNOS status 1A) or left ventricular assist device implantation. Post-transplant vital status was assessed from the heart transplant clinic records.
Statistical Analysis
Descriptive statistics are presented as mean ± SD for continuous variables and as number and percentage for categorical variables. To assess bivariate associations between clinical characteristics and spirometric parameters, we used unpaired t tests for binary characteristics and Pearson correlation for continuous characteristics. The association of spirometric parameters with the primary and secondary end points was examined with Cox proportional hazards models. We examined the appropriate functional form for the association of spirometric parameters with outcomes (linear vs nonlinear forms) using fractional polynomials and restricted cubic splines as described by Royston and co-workers.30 The validity of the proportional hazards assumption was tested with Schoenfeld residuals. For the primary end point, subjects who did not die or receive a left ventricular assist device were censored as alive at the time of heart transplantation or end of follow-up. For the secondary end point, subjects who did not die, receive a left ventricular assist device, or receive urgent heart transplantation were censored as alive at the time of non-urgent heart transplantation or end of follow-up. To examine for significant modification effects (interactions), we introduced appropriate interaction terms in the models. We also examined the association of the various ventilatory patterns with outcomes using a normal ventilatory pattern as the reference category. Finally, we searched for differences in outcomes between subjects with and without spirometric data available. The effect of baseline spirometry on post-transplant survival was examined with appropriate Cox models. A 2-sided P ≤ .05 was considered statistically significant. All analyses were performed with Stata 13 (StataCorp, College Station, Texas).
Results
Subject Characteristics and Spirometric Parameters
The mean age of subjects with spirometric variables (n = 187) was 53.1 ± 10.3 y; 32 (17.1%) were women; 130 (69.5%) were white, and 54 (28.9%) were black; and ejection fraction at time of listing was 14.9 ± 7.5%. The complete baseline subject characteristics are presented in Table 1, and the baseline central hemodynamics of subjects are presented in Table 2. Absolute and percent-of-predicted FEV1 were 2.4 ± 0.7 L and 67.3 ± 16.2, respectively. Absolute and percent-of-predicted FVC were 3.2 ± 0.9 L and 72.0 ± 16.3, respectively. Mean FEV1/FVC was 0.74 ± 0.11. White subjects had higher FEV1 and FVC compared with non-white subjects (FEV1 of 2.5 ± 0.7 L in white subjects vs 2.1 ± 0.5 L in non-white subjects, P < .001; FVC of 3.4 ± 1.0 L in white subjects vs 2.7 ± 0.7 L in non-white subjects, P < .001) and lower FEV1/FVC (0.73 ± 0.09 vs 0.77 ± 0.13, respectively, P = .02). A normal spirometric ventilatory pattern was observed in 47 (25.1%) subjects, an obstructive pattern was observed in 8 (4.3%) subjects, a restrictive pattern was observed in 95 (50.8%) subjects, and a mixed pattern was observed in 37 (19.8%) subjects. The average maximum oxygen consumption on cardiopulmonary exercise testing was 11.9 ± 2.7 mL/kg/min.
Baseline Subject Characteristics
Baseline Subject Central Hemodynamics
Effect of Clinical Variables and Medications on Spirometric Parameters
No difference was observed in spirometric variables between subjects with different clinical characteristics, smoking, lung disease, and diabetes mellitus (data not shown). Also, there was no difference in spirometric variables in subjects receiving angiotensin-converting enzymes or angiotensin II receptor blockers, aldosterone, or β blockers. However, there was a difference in subjects receiving inotropic agents. Specifically, in subjects receiving inotropes versus those not receiving inotropes, percent-of-predicted FVC was 68.8 ± 16.3 versus 74.2 ± 16.0 (P = .03), percent-of-predicted FEV1 was 64.6 ± 15.2 versus 69.1 ± 16.7 (P = .064), FEV1 was 2.3 ± 0.7 L versus 2.4 ± 0.7 L (P = .18), FVC was 3.1 ± 0.9 L versus 3.3 ± 1.0 L (P = .16), and FEV1/FVC was 0.74 ± 0.13 versus 0.75 ± 0.08 (P = .80). There was no association between diuretic dose and spirometric variables or maximum oxygen consumption (data not shown).
Outcomes
The median follow-up until death, left ventricular assist device implantation, or heart transplantation was 5.5 months (interquartile range of 2.3–15.2); the total follow-up was 223 person-years. There were 19 deaths, 28 left ventricular assist device implantations, and 74 urgent transplantations for a total of 47 primary end point events (25.1%) and 121 secondary end point events (64.7%). The annual event rate was 21.0% (95% CI 15.8–28.0) for the primary end point and 54.1% (95% CI 45.3–64.6) for the secondary end point.
Association of Spirometry With Outcomes
There was no association between spirometric parameters and outcomes in our population in both primary and secondary analyses (Table 3). The hazard ratios of FEV1 and percent-of-predicted FEV1 were 0.93 (95% CI 0.61–1.41, P = .72) and 1.00 (95% CI 0.98–1.02, P = .80), respectively, in primary analysis and 0.94 (95% CI 0.72–1.21, P = .62) and 1.00 (95% CI 0.99–1.01, P = .75), respectively, in secondary analysis. Similarly, the hazard ratios of FVC and percent-of-predicted FVC were 0.90 (95% CI 0.65–1.25, P = .52) and 0.99 (95% CI 0.98–1.01, P = .53), respectively, in primary analysis and 0.92 (95% CI 0.76–1.13, P = .43) and 1.00 (95% CI 0.99–1.01, P = .58), respectively, in secondary analysis. The fractional polynomial and restricted cubic spline models did not reveal nonlinear associations between spirometric parameters and outcomes.
Association Between Spirometric Parameters and Outcomes in Subjects Listed for Heart Transplantation
Association of Spirometry With Central Hemodynamics
There were significant correlations between central hemodynamics and spirometric variables. Elevated pulmonary artery and right ventricular systolic pressures were significantly associated with lower absolute and percent-of-predicted FEV1 and FVC (Table 4). Similarly, elevated pulmonary-capillary wedge and central venous pressures were negatively correlated with absolute and percent-of-predicted FEV1 and FVC, whereas cardiac output was positively correlated with absolute and percent-of-predicted FEV1 and FVC. FEV1/FVC was not correlated with hemodynamics.
Association Between Spirometric Parameters and Central Hemodynamics
Association of Ventilatory Patterns With Outcomes
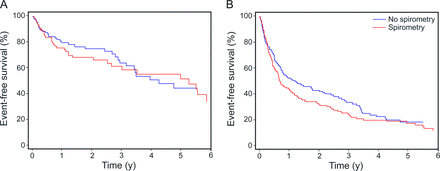
Using a normal ventilatory pattern as the reference, an obstructive, restrictive, or mixed ventilatory pattern did not confer increased risk for the primary or secondary end points. Specifically, for the primary end point, the hazard ratios of obstructive, restrictive, and mixed patterns were 0.57 (95% CI 0.12–2.64, P = .47), 1.10 (95% CI 0.55–2.18, P = .80), and 1.09 (95% CI 0.46–2.60, P = .84), respectively, with normal respiratory pattern as the reference (Fig. 1A). For the secondary end point, the hazard ratios of obstructive, restrictive, and mixed patterns were 0.68 (95% CI 0.24–1.98, P = .48), 1.48 (95% CI 0.94–2.33, P = .94), and 1.54 (95% CI 0.89–2.67, P = .89), respectively (Fig. 1B).
Survival of subjects listed for heart transplantation with different spirometric ventilatory patterns. A: Kaplan-Meier estimates for the primary outcome (death and left ventricular assist device implantation). B: Kaplan-Meier estimates for the secondary outcome (death, urgent transplantation, and left ventricular assist device implantation).
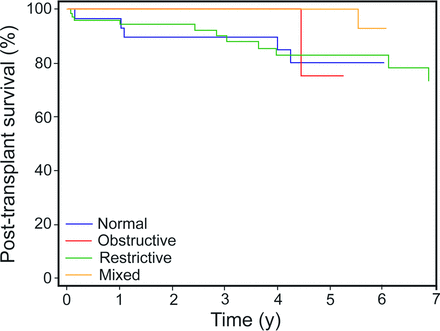
Spirometry and Post-Transplant Survival
Perioperative 30-d mortality was 1.4%, and 1-y post-transplant survival was 97.1%. Baseline spirometry was not associated with post-transplant survival (Fig. 2). At 1 y, survival was 100%, 96.0%, 100%, and 96.5% for subjects with baseline obstructive, restrictive, mixed, and normal spirometric patterns, respectively (log-rank chi-square = 2.80, P = .42).
Post-transplant survival according to baseline spirometric pattern.
Outcomes in Subjects Without Spirometric Data
The median follow-up of the 158 subjects without available spirometric data was 7.2 months (interquartile range of 2.4–26.4); total follow-up was 248 person-years. Among these subjects, there were 47 (26.6%) primary outcome events and 101 (63.9%) secondary outcome events. The annual event rates were 17% (95% CI 12.5–23.0) for the primary outcome and 40.8% (95% CI 33.6–49.6) for the secondary outcome. There was no difference in outcomes between those subjects with and without spirometric data (P = .54 and P = .15, respectively) (Fig. 3).
Survival of subjects listed for heart transplantation with and without spirometry. A: Kaplan-Meier estimates for the primary outcome (death and left ventricular assist device implantation). B: Kaplan-Meier estimates for the secondary outcome (death, urgent transplantation, and left ventricular assist device implantation).
Discussion
The main observation of our study is that spirometric variables were not associated with outcomes in subjects with advanced heart failure listed for heart transplant. Similarly, we did not observe any prognostic association of obstructive, restrictive, or mixed ventilatory pattern with outcomes. To our knowledge, this is the first study reporting the association between spirometric parameters and outcomes in this population. Studies investigating the prognostic value of spirometry in subjects with acute25 or stable24,26 heart failure showed that spirometric variables predict outcomes in these stage C heart failure populations. However, we did not observe a similar association in our study, in which we included subjects with stage D heart failure exclusively.
There are several potential explanations for our findings. The population of patients with heart failure listed for heart transplantation is selected to be free of severe end-organ dysfunction, which may considerably attenuate the prognostic value of spirometry. Impaired cardiac function is the initiating process for heart failure, and as the disease progresses, the patients enter a state of systemic illness that impacts multiple organ systems and finally leads to advanced disease. These heart failure-induced changes include also changes in the pulmonary system. The pulmonary and cardiac systems are intimately linked anatomically and physiologically; because of this, changes in the cardiac system have profound effects on the pulmonary system, also causing abnormalities in spirometric parameters. However, these spirometric abnormalities might be just indicators of the heart-lung relationship in this specific population of heart failure patients without underlying prognostic importance. It has been reported that spirometry is not useful for diagnosis and grading of pulmonary diseases in subjects with heart failure undergoing heart transplantation because of this heart-lung relationship.31 This may be the case for prognostic purposes as well.
Previous studies showed that subjects with heart failure often develop significant abnormalities in pulmonary function12,14,15,32 that range from relatively marginal dysfunction to more significant spirometric abnormalities, both restrictive and obstructive.14,33 In our population, pulmonary function was impaired in 75% of subjects, whereas only a small proportion had a history of chronic lung disease, confirming the association of heart failure with reduced pulmonary function and the findings of previous studies that a high proportion of subjects with heart failure have spirometric ventilatory abnormalities.14 A higher proportion of subjects had a restrictive pattern than an obstructive or mixed pattern, which has been described in other studies as well. In fact, this imbalance of case mix of spirometric ventilatory patterns in our cohort, in conjunction with the small number of events among subjects with obstructive and mixed patterns, may be the reason for the observed lack of association of spirometric patterns with outcomes rather than the absence of biologic association. The specific mechanism or mechanisms leading to changes or no change in lung function in patients with heart failure are not completely clear. Possible mechanisms that have been suggested include chronic pulmonary congestion and hypertension,34 respiratory muscle weakness,35 low cardiac output,36 and cardiomegaly.15,32
Data on the importance of reduced spirometric values, especially FEV1, have been conflicting also. In a retrospective study of 186 out-patients with heart failure and reduced ejection fraction (mainly stage C and New York Heart Association classes I and II), only severe air-flow obstruction (FEV1 < 50% of predicted value) appeared to be a predictor of reduced survival.37 In another prospective study of 439 subjects with heart failure and reduced ejection fraction, FEV1 < 80% of predicted was associated with reduced survival.26 This underscores that more studies are needed to definitely answer the question of the importance of pulmonary function abnormalities in heart failure survival.
In our study, we observed no association between spirometric values and functional capacity of subjects listed for heart transplantation. This observation is in accordance with the findings of another study in which the influence of spirometric variables on peak exercise capacity diminished as symptoms of heart failure worsened, and there was no relation in the New York Heart Association class III–IV subjects.38 We also observed a significant association of spirometric variables and central hemodynamics, in accordance with previous studies.39,40 Increased pressures in the pulmonary circulation have been associated with bronchial obstruction, leading to pulmonary function abnormalities.41 With medical therapy, there is significant improvement in restrictive or obstructive abnormalities, and this improvement is explained by a reduction of pulmonary pressures, with a subsequent decrease in interstitial edema and bronchial wall congestion.14,39,42
Our study has some important limitations. There were no available spirometric data on all listed subjects. However, there was no difference in the outcomes (primary and secondary) between the subjects with and without spirometric data, so the limitation of selection bias could be considered as relatively weak. We did not obtain diffusion capacity data in our subjects during their evaluation; thus, we cannot describe any difference in the prognostic value of this test in this specific heart failure population. We used fixed cutoff points for percent-of-predicted FEV1 and FVC to describe obstructive versus restrictive spirometric patterns instead of using, for example, the lower limit of normal. Finally, the case mix of the various spirometric patterns is unbalanced, and considering the small number of subjects with obstructive and mixed patterns (and the corresponding small number of events), the observed lack of association may merely reflect lack of power rather than absence of biologic association. A comparative study would be needed to evaluate whether different criteria for the various spirometric patterns would be more valuable in this group of subjects.
Conclusions
In summary, spirometric parameters were not associated with outcomes in the homogeneous group of subjects with heart failure listed for heart transplantation. Because severe heart failure leads to significant reduction in FEV1 and FVC, the majority of advanced patients with heart failure will have impaired spirometric values. Therefore, the usefulness of spirometry to diagnose and grade pulmonary function abnormalities (obstruction or restriction) in this population needs further evaluation.
Footnotes
- Correspondence: Vasiliki V Georgiopoulou MD MPH, Emory Clinical Cardiovascular Research Institute, Suite 535A, 1462 Clifton Road, NE, Atlanta, GA 30322. E-mail: vgeorgi{at}emory.edu.
This study was partially funded through an Emory University Heart and Vascular Board grant entitled “Novel Risk Markers and Prognosis Determination in Heart Failure.” The authors have disclosed no conflicts of interest.
- Copyright © 2015 by Daedalus Enterprises