Abstract
BACKGROUND: Both premature and delayed liberation from mechanical ventilation are associated with increased morbidity and mortality, and fluid balance could negatively influence extubation outcomes. We sought to determine the impact of fluid balance in the 48 h before a spontaneous breathing trial (SBT) on weaning outcomes in a mixed ICU population.
METHODS: This was a prospective observational study in 2 adult medical-surgical ICUs. All enrolled subjects met eligibility criteria for weaning from mechanical ventilation. SBT failure was defined as inability to tolerate a T-piece trial for 30–120 min. Data on demographics, physiology, fluid balance in the 48 h preceding SBT (fluid input minus output over the 48-h period), lung ultrasound findings, and outcomes were collected.
RESULTS: Of a total of 250 SBTs, SBT failure eventuated in 51 (20.4%). Twenty-nine subjects (11.6%) had COPD, and 40 subjects (16%) were intubated due to respiratory sepsis. One-hundred eighty-nine subjects (75.6%) were extubated on the first attempt. Compared with subjects with SBT success, SBT failure subjects were younger (median of 66 vs 75 y, P = .001) and had a higher duration of mechanical ventilation (median of 7 vs 4 d, P < .001) and a higher prevalence of COPD (19.6 vs 9.5%, P = .04). There were no statistically significant differences in 48-h fluid balance before SBT between groups (SBT failure, 1,201.65 ± 2,801.68 mL; SBT success, 1,324.39 ± 2,915.95 mL). However, in the COPD subgroup, we found a significant association between positive fluid balance in the 48 h before SBT and SBT failure (odds ratio of 1.77 [1.24–2.53], P = .04).
CONCLUSIONS: Fluid balance should not delay SBT indication because it does not predict greater probability of SBT failure in the medical-surgical critically ill population. Notwithstanding, avoiding positive fluid balance in patients with COPD might improve weaning outcomes. (ClinicalTrials.gov registration NCT02022839.)
Introduction
Imprecise definition of weaning from mechanical ventilation prevents the rigor of research in this area and interpretation of findings.1 Both premature and delayed discontinuation of mechanical ventilation have been associated with increased mortality (36%),2 assuming that re-intubation is not related to upper-airway obstruction.3 Early identification of patients who are able to breathe spontaneously results in a shorter duration of mechanical ventilation and lower complication rates.4 Incidence of ventilator-associated pneumonia is estimated to be 1–3% each day on mechanical ventilation.5
Switching a patient from positive-pressure ventilation to spontaneous breathing reestablishes negative inspiratory intrathoracic pressure, thus increasing venous return (left-ventricular preload), central blood volume, and left-ventricular afterload. This normal condition, often an effort test for the patient, can decompensate cardiorespiratory function in cases of volume overload and left-ventricular systolic or diastolic dysfunction.6 Also, in patients with preexisting right-ventricular disease, an increase in weaning-induced right-ventricular afterload may occur as a consequence of hypoxemia or worsening of intrinsic PEEP, especially in the COPD population.7 In addition to a simultaneous increase in systemic venous return, higher right-ventricular afterload may lead to marked right-ventricular enlargement during weaning, thus impeding the diastolic filling of the left ventricle through a biventricular interdependence mechanism.
Previous observational studies8–10 on weaning procedures found a correlation between higher fluid balance and extubation failure. However, there was considerable diversity in terms of populations evaluated, weaning and extubation protocols, and outcomes analyzed. Furthermore, spontaneous breathing trials (SBTs) were not discriminated among outcomes. A randomized controlled trial showed that fluid management guided by brain natriuretic peptide plasma concentrations reduced time to weaning,11 despite similar fluid balance between groups on the day of extubation. Empirically and unnecessary administration of diuretics in every ready-to-wean patient has become more and more frequent, even though extracardiac causes are responsible for weaning failure in at least 50% of cases, and ∼70% of patients are successfully extubated on the first attempt (simple weaning). Moreover, uncontrolled diuretic therapy may have potentially harmful effects, such as serious electrolyte disturbances and microatelectasis related to bronchiolar obstruction by dry bronchial secretions.7
We therefore hypothesized that fluid balance should not delay the decision to submit the ordinary mechanically ventilated patient to an SBT because it cannot accurately predict the earliest time that a individual might resume spontaneous breathing. The objective of our study was to prospectively assess the variables associated with SBT failure in a heterogeneous group of mechanically ventilated subjects.
QUICK LOOK
Current knowledge
Early and delayed discontinuation of mechanical ventilation are associated with significant morbidity and mortality. Delayed discontinuation may result from insufficient screening processes or failure to recognize disease resolution. A positive fluid balance may impact resolution of lung disease, diminish lung compliance, and delay weaning.
What this paper contributes to our knowledge
Fluid balance in the preceding 48 h before extubation did not impact weaning outcomes in a group of medical-surgical subjects. Subjects with COPD appeared to benefit from fluid restriction before extubation.
Methods
Between January 2011 and March 2013, nonconsecutive subjects > 18 y of age who had undergone invasive mechanical ventilation for 24 h were enrolled from mixed ICUs in 2 private hospitals. Patients with a tracheostomy were excluded. The research ethics board at each center approved the study and waived the requirement for informed consent. The study was registered as NCT02022839 at ClinicalTrials.gov.
Subjects were assessed daily for eligibility to wean according to: (1) improvement in underlying condition that led to acute respiratory failure, (2) alertness and ability to communicate, (3) adequate gas exchange as indicated by a PaO2 of at least 60 mm Hg with an FIO2 of < 0.40, (4) rapid shallow breathing index of ≤ 105, and (5) vasoactive drugs at low and stable doses (norepinephrine doses of < 0.12 μg/kg/min or equivalent dopamine doses).
The main outcome of interest was SBT failure, defined as inability to tolerate a T-piece trial of spontaneous breathing for 30–120 min, in which case, subjects were not extubated. The breathing trial was interrupted if the subject developed signs of respiratory discomfort (breathing frequency of > 38 breaths/min, arterial oxyhemoglobin saturation of < 90%, use of accessory respiratory muscles, or paradoxical thoracoabdominal ventilation), tachycardia (heart rate of > 130 beats/min), hemodynamic instability (systolic blood pressure of < 90 mm Hg or 20% over basal levels), or change in mental status (drowsiness, coma, anxiety).
Demographic data, including age, sex, race, comorbidities, severity of illness at the time of ICU admission, reason for the initiation of mechanical ventilation, physiological weaning predictors, last chest radiograph findings available before SBT, and fluid balance in the 48 h preceding SBT, were recorded. The presence of diastolic or systolic left-ventricular dysfunction (the latter condition defined as an ejection fraction of < 45%) was documented according to echocardiogram reports dated up to 6 months before admission. Diagnosis of COPD was based on history, physical examination, chest radiograph, and previous pulmonary function tests.
Fluid balance was routinely recorded on report sheetsand was defined as total input minus total output (tallied daily at midnight). Losses via urinary, gastrointestinal, or other drainage tubes were subtracted from all fluids, nutrition, medications, and blood products administered, whatever the route of administration.
Statistics
On the basis of the results of Upadya et al,8 who observed a 1,500-mL higher fluid balance in the SBT failure group compared with the SBT success group in the 48 h before extubation, we estimated that 250 subjects would have 91% power to show the same difference at a 2-sided α level of 0.05. Results are expressed as the mean ± SD, median and interquartile range, and proportions as appropriate. The normal distribution of the various parameters was investigated observing the distribution of data and using the Kolmogorov-Smirnov test. We used the Student t test or the Mann-Whitney U test to compare continuous variables, and the chi-square test or the Fisher exact test to compare proportions as appropriate. The primary end point was also analyzed in the 3 predefined subgroups: COPD, left-ventricular systolic dysfunction (defined as an ejection fraction of < 45%), and isolated left-ventricular diastolic dysfunction. Receiver operating characteristic curves were generated for these subgroups, comparing their ability to discriminate between SBT success and failure subjects according to fluid balance values. Finally, all subjects were divided into categories according to fluid balance in the 48 h before SBT using arbitrary steps of 1,000 mL as described by Frutos-Vivar et al.10
Results
We obtained complete data in 250 weaning procedures. Overall, SBT failure occurred in 51 subjects (20.4%). Table 1 shows the baseline characteristics of the study cohort according to outcome. Subjects who were successfully extubated were older (median of 75 vs 66 y, P = .001) and had been intubated for a shorter duration (mechanical ventilation median of 4 vs 7 d, P < .001). There was also a lower prevalence of COPD in the SBT success group (19.6 vs 9.5%, P = .04).
Characteristics of the Study Cohort
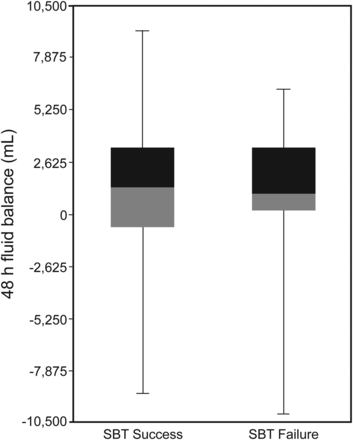
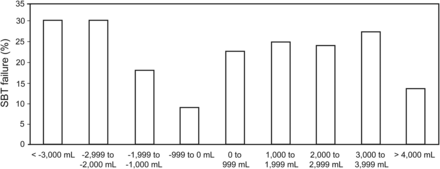
Fluid balance in the 48 h preceding SBT was similar between groups (1,324.39 ± 2,915.95 vs 1,201.65 ± 2,801.68 mL for the SBT success and failure subjects, respectively; P = .52) (Fig. 1). Using arbitrary steps of 1,000 mL, the prevalence of SBT failure demonstrated a random distribution among categories (Fig. 2).
Forty-eight hours fluid balance according to spontaneous breathing trial (SBT) outcomes (P = .52 between SBT success and failure). Box plots denote the median and interquartile range; points show the maximum and minimum for each group.
Prevalence of weaning failure by fluid balance category. Subjects were divided into categories using arbitrary steps of 1,000 mL. Columns depict the spontaneous breathing trial (SBT) failure rate for each category. Note its random distribution, with no more preponderance of SBT failure in either positive or negative fluid balance values.
The results from subgroup analysis of subjects with COPD, left-ventricular systolic dysfunction, and isolated left-ventricular diastolic dysfunction are shown in Table 2. An area under the receiver operating characteristic curve of 0.70 (0.50–0.89) was found in subjects with COPD with a cutoff of 0 mL to SBT failure. Therefore, comparing individuals with 48-h fluid balance above and below the 0-mL cutoff according to SBT outcomes resulted in a significant association between positive fluid balance in the 48 h preceding SBT and SBT failure in this subgroup (odds ratio of 1.77 [1.24–2.53], P = .04), as shown in Figure 3. The areas under the receiver operating characteristic curve were 0.59 and 0.50 for left-ventricular systolic dysfunction and isolated left-ventricular diastolic dysfunction, respectively.
Fluid Balance and Outcomes According to Specific Subgroups
Association between positive fluid balance in the 48 h preceding T-piece testing and spontaneous breathing trial (SBT) failure in subjects with COPD (odds ratio of 1.77 [1.24–2.53], P = .04).
Discussion
In a heterogeneous cohort of mechanically ventilated subjects who were candidates for SBT, we found no association between fluid balance in the preceding 48 h and SBT outcomes. However, subjects with COPD might derive some benefit in terms of not allowing positive fluid balance, probably due to heart-lung interaction issues.
Despite the relationship between failure to wean from mechanical ventilation and positive fluid balance, there is no clear evidence of principle cause and effect, and outcomes employed are diverse among studies (weaning, extubation, or both). A central question for clinicians is whether extubation failure is simply a marker of poor prognosis or instead contributes to induce a poor prognosis.12 A retrospective analysis of the Vasopressin and Septic Shock Trial (VASST) demonstrated that mortality increases with positive fluid balance in a linear manner independent of severity of illness or shock, and this dose-response correlation was found only 12 h after study enrollment.13 Whether fluid balance independently affects outcome or is just a confounder (a marker of severity of illness) remains unclear, but clearly, aggravating fluid balance by using the wrong tools and the wrong end points should not take place in the context of current knowledge.
Inaccuracies in monitoring and recording fluid therapy in daily practice are a growing issue in the intensive care setting due to the lack of agreement for standardized body weight measurements.14–16 All of the studies neglected to consider nutritional aspects, and some did not take into account the sensible or insensible fluid losses. Average daily and cumulative fluid balances were arithmetically incorrect in about one third of cases.15 We could even question the clinical importance or significance of changes in total body water, as the volume load of specific compartments (intravascular volume, cardiac preload, extravascular lung water) is probably more relevant.
Upadya et al8 examined 87 subjects who underwent 205 breathing trials (T-piece or pressure support) between 2002 and 2003 and verified that negative fluid balance in the preceding 24 or 48 h and the net negative cumulative balance were associated with weaning success. The sample contained a high percentage (46%) of subjects with COPD, and only 44% was classified as simple to wean. Although administration of diuretics was associated with negative fluid balance, it was not associated with weaning outcomes. A prospective study of 40 trauma and surgical subjects who were at least 60 y old also proposed a direct relationship between negative fluid balance and weaning success; nevertheless, the weaning failure group already had a fluid balance significantly higher at entry into the study.9 Frutos-Vivar et al10 published a representative cohort study of 900 subjects, searching for risk factors predicting extubation failure following an SBT and concluded that re-intubated subjects were more likely to have a positive fluid balance in the 24 h before extubation. Finally, a randomized controlled trial showed that fluid management guided by brain natriuretic peptide plasma concentrations reduced time to weaning,11 a benefit observed predominantly in subjects with left-ventricular systolic dysfunction in a subgroup analysis, regardless of the lack of a significant difference in fluid balance between the standard-care and intervention groups on the day of extubation.
A comparison of our study design with the others mentioned above is shown in Table 3. Note our higher prevalence of simple weaning, as described in literature, and use of SBT failure as an outcome instead extubation failure. We chose SBT failure as principal outcome because we aimed to predict the earliest time that a patient might resume spontaneous breathing. Moreover, the exact reason for extubation failure often escapes identification. Re-intubation is usually performed because of an apparently new episode of respiratory distress, which may be related to primary respiratory failure, congestive heart failure, aspiration, ineffective cough with airway secretion buildup, or upper-airway obstruction. Other reasons for re-intubation include the onset of new sepsis, surgical complications, acute coronary syndrome, and neurological impairment. This multiplicity of causative factors helps to explain the clinical difficulties raised by extubation and the persistent uncertainties about the pathophysiology of extubation failure.1
Comparison of Studies
Limitations of our study include observational design, with all its intrinsic methodological flaws, and small sample size with a high prevalence of elderly population and a lower prevalence of COPD compared with other similar studies. Also, there is potential for inaccuracy and lack of precision of clinical data retrieved from daily flow sheets.
On January 28, 2013, we received 10 patients who were rescued from the Kiss nightclub fire in Santa Maria, Rio Grande do Sul, Brazil. They were between 17 and 23 y of age and had smoke inhalation injury. Six patients failed the T-piece test at least once. By excluding these patients, there would be no statistically difference in age (P = .21) or fluid balance (P = .52) between SBT failure and success groups.
Conclusions
In summary, fluid balance may not predict SBT outcomes in a mixed medical-surgical ICU population.
Footnotes
- Correspondence: Ana Carolina Peçanha Antonio MD, 11 Ari Marinho, Apartment 210, Porto Alegre 90520-300, Brazil. E-mail: ana.carolina.antonio{at}gmail.com.
The authors have disclosed no conflicts of interest.
See the Related Editorial on Page 1213
- Copyright © 2015 by Daedalus Enterprises