Abstract
BACKGROUND: Children with cystic fibrosis may have a deficiency of micronutrients, including zinc, which may affect their susceptibility to infections. There is a paucity of data on zinc supplementation among children with cystic fibrosis. We hypothesized that a pharmacologic dose of zinc administered daily for 12 months would reduce the need for antibiotics by 50%.
METHODS: This double-blind randomized placebo-controlled trial was conducted among children with cystic fibrosis to assess the effect of zinc supplementation on the need for antibiotics and pulmonary function tests. The children, age 5–15 y, of either sex, received either 30-mg zinc tablets or similar looking placebo tablets daily in addition to standard care. They were followed up every month for a period of 12 months and whenever they had pulmonary exacerbations. Their serum zinc was estimated at baseline and at 12 months of enrollment. During each visit, the children underwent a pulmonary function test and sputum culture.
RESULTS: Of a total of 43 children screened, 40 were enrolled, and of them, 37 completed the study. The median (interquartile range) number of days of the administration of antibiotics over 12 months of follow-up among the children receiving zinc was 42 (14–97) d. In the placebo group, it was 38 (15–70) d (P = .79). There were no significant differences in the percent-of-predicted FEV1 or change in FEV1 values at 12 months (P = .44). The number of children in whose respiratory specimens Pseudomonas was isolated was similar for the 2 groups at different time intervals. The adverse events reported were similar in the 2 groups.
CONCLUSION: We did not find any significant difference in the need for antibiotics, pulmonary function tests, hospitalization, colonization with Pseudomonas, or the need for antibiotics for children with cystic fibrosis receiving zinc supplementation of 30 mg/d.
Introduction
Cystic fibrosis is the most common life-limiting inherited disease. In the 1980s, it was considered a disease of the white population. Over the years, cystic fibrosis has been reported from different parts of the world, including Asian countries.1 The major cause of morbidity among children with cystic fibrosis is repeated lung infections.
Children with cystic fibrosis have been reported to suffer from micronutrient deficiencies.2 They are more prone to illnesses caused by zinc deficiencies, such as acrodermatitis, which improve with supplementation.3–5 Deficiency of micronutrients, including zinc, is associated with an increased risk of infection. However, data on zinc supplementation among children with cystic fibrosis, although limited, suggest that the results of supplementation are variable in terms of improvement in pulmonary function test or reduction in the need for antibiotics.6–9 Almost a quarter of adults with cystic fibrosis who had a good nutritional status had a low plasma zinc concentration, and those with a low plasma zinc concentration had a poor clinical outcome.10 Zinc deficiency is widespread among Indian children. There are no studies on zinc supplementation from resource-limited settings. We hypothesized that therapeutic zinc supplementation might reduce pulmonary exacerbations and improve FEV1 among children from resource-poor settings, where zinc deficiency may be more prevalent. We aimed to assess the efficacy of zinc supplementation among children with cystic fibrosis.
QUICK LOOK
Current knowledge
Children with cystic fibrosis have been reported to suffer from micronutrient deficiencies. They are more prone to illnesses caused by zinc deficiencies, such as acrodermatitis, which improve with supplementation. Deficiency of micronutrients, including zinc, is associated with an increased risk of infection.
What this paper contributes to our knowledge
In this double-blind randomized placebo-controlled, study of zinc supplementation (30 mg/d) in a small group of subjects with cystic fibrosis, there were no differences in the need for antibiotics, pulmonary function tests, hospitalization, colonization with Pseudomonas or the need for antibiotics. Zinc levels were low in all subjects before study enrollment and increased during therapy.
Methods
This double-blind randomized placebo-controlled trial was carried out at a tertiary care hospital in northern India. Children with cystic fibrosis were the subjects of the study, which was approved by the institutional ethics committee and registered with the Clinical Trials Registry, India (CTRI/2011/12/002230).
Children of either sex, age 5–15 y and with cystic fibrosis, were enrolled in the study. Cystic fibrosis was diagnosed by 2 abnormal sweat chloride values in the presence of clinical symptoms. Children who received zinc supplementation in the month prior to study enrollment and those not willing to report for regular follow-up were excluded.
After obtaining informed consent, the baseline history was recorded, and an examination was done on all of the children. Spirometry was performed, and sputum or postphysiotherapy throat swabs were cultured. A blood sample was taken for the estimation of zinc and stored at −20°C.
All enrolled children were followed up every month for a period of 12 months. The children were asked to report if there was any pulmonary exacerbation. On each visit, a clinical examination, spirometry, and sputum/cough swab cultures were performed. The serum zinc levels were estimated again at the end of 12 months of follow-up.
Sample Size
The only randomized controlled trial that has evaluated the effect of zinc on the duration of antibiotic use among children with cystic fibrosis showed a reduction of >50% in zinc-deficient children.7 The average number of annual days of systemic antibiotics for cystic fibrosis subjects is 56 ± 30 d (unpublished data). We hypothesized that zinc supplementation would reduce the average duration of antibiotics to 28 ± 15 d. To be able to determine the effect of the treatment with 95% CI and 90% power, 16 subjects were required in each group. We expected that 90% of children with cystic fibrosis would be zinc-deficient, so we planned to enroll a total of 40 children to identify 32 zinc-deficient subjects after accounting for a 10% loss to follow-up.
Outcomes
The primary outcome variable was a reduction in the average days of systemic antibiotics in the zinc group as compared with placebo. The secondary outcomes were an improvement in FEV1 and a difference in the rate of colonization with Pseudomonas in the zinc and placebo groups.
Randomization
Block randomization was done by a person not involved in the study. The drugs were labeled by a person not involved in data collection and data analysis. The strips of tablets (zinc/placebo) were serially numbered as per the randomization list.
Blinding
Similar looking zinc or placebo tablets were administered to the children.
Intervention
All enrolled subjects received the study drug daily for 12 months. The study drug was a dispersible tablet, containing 20 mg of zinc or placebo (supplied by Bharat Immunologicals and Biologicals [Bulandshahr, India], a Government of India undertaking). The parents were asked to dissolve one-and-a-half tablets (ie, 30 mg of zinc) in 5–10 mL of water and administer this to their children every day. If a child vomited out the tablet within 30 min of its administration, the parents were asked to repeat the dose. The subjects were supplied with 50 tablets during each visit. They were asked to bring the remaining tablets on their next visit after 4 weeks.
Co-interventions
All enrolled children continued to receive standard treatment, including supplementation with enzymes, fat-soluble vitamins, salt, and potassium chloride. They were prescribed antibiotics. A subject was considered to have pulmonary exacerbation if he/she had any 4 of the following: (1) change in the volume or color of sputum; (2) new or increased hemoptysis; (3) increased cough; (4) increased dyspnea; (5) increased malaise, fatigue, or lethargy; (6) temperature >38°C; (7) anorexia or weight loss; (8) sinus pain or tenderness; (9) change in sinus discharge; (10) change in the physical findings on examination of the chest; (11) decrease in pulmonary function by 10% or more; and (12) radiographic changes.11
Adherence
Adherence to the intervention was assessed on the basis of the pill count. The parents were asked to make entries in a diary on all of the medications administered, including the study drug.
Laboratory Techniques
Serum zinc (baseline, 12 month) levels were determined with a flame furnace atomic absorption spectrophotometer (GBC Avanta, Dandenong, Australia), using standard techniques, and with SERONORM (Sero, Billingstad, Norway). The limits of detection for zinc were 10–160 μg/dL. For quality control, pooled serum with a known value was used. The interassay variability was <10%. A serum zinc concentration level of <65 μg/dL was taken as a zinc-deficient state.12
Microbiology
The respiratory specimens were cultured on sheep blood agar, MacConkey agar, chocolate agar, and Burkholderia cepacia selective agar (Hi Media, Mumbai, India). Growth was identified by conventional microbiological methods. The test for antimicrobial susceptibility was carried out using the Kirby-Bauer method and interpreted according to the guidelines of the Clinical and Laboratory Standards Institute.13
Data Analysis
A double data entry was made in the Microsoft Access program (Microsoft Corp., Richmond, Washington) every week. Intention-to-treat as well as per protocol analysis were performed using STATA software, Stata 11.1 (StataCorp LP, College Station, Texas). The summary statistics of continuous variables are reported as mean ± SD or median (interquartile range), depending on whether the distribution was normal or skewed; parametric and non-parametric tests were used, respectively. For continuous variables, the Student t test was used to determine the significance. Proportions were compared using the chi-square test.
A data safety and monitoring board was constituted, and the study was started after obtaining permission from this board. The data were regularly presented to the data safety and monitoring board.
Results
In this double-blind randomized placebo-controlled trial, we recruited a total of 40 eligible subjects between December 2011 and July 2012 and followed them for a minimum of 12 months until July 2013. Of the 40 children, 20 each were randomized to receive zinc supplementation or placebo. Two subjects from the zinc group and 1 from the placebo group withdrew consent, leaving 18 and 19 subjects in the zinc and placebo groups, respectively. A total of 37 children completed 12 months of follow-up (Fig. 1).
Flow chart.
Baseline Characteristics
Baseline characteristics of the subjects in the 2 groups were similar (Tables 1 and 2). Mean ± SD serum zinc levels at baseline were 51.92 ± 15.76 and 48.90 ± 13.47 μg/dL in the zinc and placebo groups, respectively; at 12 months, the levels were 58.51 ± 10.28 and 57.62 ± 12.74 μg/dL, respectively (P = .92).
Baseline Characteristics of the Subjects (N = 40)
Anthropometric Indices at the Completion of the Study (at 12 Months)
Primary Outcome
The median (range) number of days of administration of oral or intravenous antibiotics over 12 months' follow-up for children receiving zinc was 42 (0–238) d. The corresponding figure for children receiving placebo was 38 (0–224) d (P = .79) (Table 3).
Need for Antibiotics for Pulmonary Exacerbations
Spirometry
Table 4 shows the serial changes in the percent- of-predicted FEV1 during the study period. There were no statistically significant differences in the percent-of-predicted FEV1 values at 12 months or in the change in percent-of-predicted FEV1 during the 12-month study period.
Pulmonary Function Test at Different Time Intervals
Microbiology
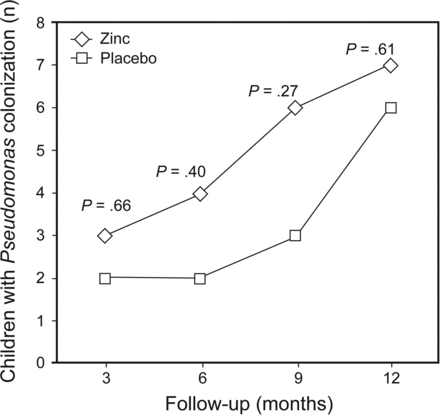
The number of children found to have growth of Pseudomonas aeruginosa in their sputum/throat swab was similar in the 2 groups at different time intervals (Fig. 2). No subject was culture-positive for B. cepacia.
Pseudomonas infection at different time intervals in groups administered placebo and zinc supplementation.
Adherence to Treatment
Only 3 subjects did not complete the study. Among the 37 children who completed the study, the adherence to the trial medication was >95%.
Adverse Events
A total of 15 serious adverse events or hospitalization occurred in 13 children during the study period. All of the serious adverse events related to pulmonary exacerbations, which are known to occur in cystic fibrosis; these were successfully managed. Seven (38.9%) children in the zinc group required hospitalization, compared with 6 (31.57%) in the placebo group (P = .64). None of the enrolled subjects died during the study period.
Discussion
In this randomized controlled trial, we did not find any significant difference in the need for antibiotics, intravenous antibiotics, hospitalization or colonization with Pseudomonas among children with cystic fibrosis receiving zinc supplementation of 30 mg daily as compared with those receiving placebo.
There is a paucity of data on the role of zinc in cystic fibrosis. It is thought that steatorrhea causes loss of zinc in the stool and should lead to low zinc levels among children with cystic fibrosis. However, no such relation was found in the study on 36 children with cystic fibrosis by Van Biervliet et al.14 Another study reported that serum zinc levels in children with cystic fibrosis were similar to those of normal controls but that children with lower serum zinc levels had significantly worse results on the pulmonary function tests.15 An increase has been observed in the sputum zinc levels in pulmonary exacerbations, and a decrease has been observed in the levels after treatment in bronchiectasis (cystic fibrosis and non-cystic fibrosis bronchiectasis); however, the significance of this remains unclear.16
Tinley et al17 observed an interaction between the serum zinc and vitamin levels. They reported an improvement in the zinc levels after supplementation with retinol.
The interaction of zinc and the airways in cystic fibrosis is not clearly understood. It is suggested that the antioxidant action of zinc and vitamin C may affect the flux of glutathione across cells and modify the rheology of sputum in cystic fibrosis.18,19
Data on zinc supplementation among children with cystic fibrosis are limited to 4 studies.6–9 Safai-Kutti et al,9 in their placebo-controlled double-blind crossover study in children with cystic fibrosis (N = 13, age 2 y, 3 months to 19 y, 1 month), gave zinc supplementation and placebo to the subjects for 6 months. They observed that all subjects had low plasma zinc levels at baseline. They reported no changes in the clinical status, lung function, or growth velocity in response to zinc or placebo.9
Abdulhamid et al7 conducted a double-blind randomized controlled trial in which 26 children were treated with placebo or zinc (30 mg/d) for 1 y. They documented that the requirement for antibiotics was significantly lower among those receiving zinc than those receiving placebo (P = .05). Improvement was more pronounced among those who had low plasma zinc at baseline (P = .02). Reduced levels of plasma interleukin-6 and interleukin-8 were also documented.7
In a retrospective review of 21 subjects, Van Biervliet et al6 reported a decrease in pulmonary exacerbations, from 3 to 2 (P < .02), as well as an increase in the FEV1 from 72.0 to 76.5% (P < .02). They also observed an improvement in weight for height, from 90 to 94% (P = .043). The median age of the study subjects was 8.9 y, and they received zinc sulfate in doses of 5 mg/kg (maximum 150 mg).6
Ataee et al8 studied 30 children with cystic fibrosis (mean age: 8.65 ± 3.01 y), who were administered zinc at a dosage of 2 mg/kg/d for 6 months, along with standard therapy. They measured the subjects' clinical parameters before and after the administration of zinc. They observed a significant improvement in the height, weight, and body mass index in those who received zinc and had a normal pulmonary function test. The improvement was not evident among those with a low pulmonary function test. Pulmonary function in the form of percent-of-predicted FEV1 declined from 88.22 ± 24.61 to 82.86 ± 23.69% among males and from 87.29 ± 15.83 to 83.8 ± 18.4% among females.8
In our study, the reasons for no difference in the need for antibiotics and in the pulmonary function tests between the 2 groups may be explained by the following factors. The sample size was not large enough to detect the difference; we calculated the sample size on the basis of a clinically relevant difference in the number of days of antibiotic intake. Serum zinc levels improved in the placebo group as well, although the majority of the children continued to be deficient. An improvement in the serum zinc levels has also been shown in children with tuberculosis receiving placebo or zinc.20 In our study, the majority of the children had low serum zinc levels at baseline; therefore, the dose of zinc administered may not have been sufficient to have an impact on the outcomes, although we used a dose of 30 mg/d. One limitation of the study is its small sample size, whereas its strengths include 1 y of follow-up and a high rate of adherence to the study drug.
Conclusion
To conclude, zinc supplementation at a dose of 30 mg/d in children with cystic fibrosis does not improve their clinical status, need for antibiotics, or pulmonary function tests. Studies are required on larger numbers of children administered higher daily doses of zinc.
Acknowledgments
We express our gratitude to the data safety and monitoring board: Dr GR Sethi (Maulana Azad Medical College, New Delhi, India), Dr S Bhatnagar (Translational Health Sciences Institute, Faridabad, India), and Dr RM Pandey (All India Institute of Medical Sciences, New Delhi, India). We thank Mr Puneet for carrying out serum zinc estimation and microbiology culture. We are grateful to Byword Editorial Consultants (Delhi, India) for technical and language editing of the manuscript.
Footnotes
- Correspondence: Sushil K Kabra MD, Department of Pediatrics, All India Institute of Medical Sciences, New Delhi 110029, India. E-mail: skkabra{at}hotmail.com.
This work was supported by the Indian Council of Medical Research, New Delhi, India. The authors have disclosed no conflicts of interest.
- Copyright © 2016 by Daedalus Enterprises