Abstract
In the past few decades, assessment of exhaled CO2 in both intubated and non-intubated patients has evolved into an essential component in many aspects of patient monitoring. Besides the basic assessment of ventilation, exhaled CO2 monitoring can provide valuable patient safety information and critical physiologic data in regard to the ventilation and perfusion matching in the lungs, cardiac output, and metabolic rate. Despite these important clinical monitoring benefits and widespread availability, exhaled CO2 monitoring is often underutilized. The purpose of this paper is to review the importance and present the extensive body of knowledge to support the use of exhaled CO2 monitoring in various areas of clinical practice. Advanced application concepts and the future development of exhaled CO2 monitoring will also be discussed.
- end-tidal CO2
- capnometry
- capnography
- capnogram
- volumetric capnography
- physiologic dead space
- resting energy expenditure
Introduction
The measurement of CO2 in air was first developed around 1918 and performed to analyze gas concentrations in mines.1 This process of analysis was painstakingly tedious, using an intricate apparatus that involved measuring quantities of CO2 and other gases that were chemically absorbed from a known gas volume. The volume of absorbed CO2 was then compared with the total gas volume, which yielded the fraction or percentage of CO2 present. A decade later, a technique was developed to analyze exhaled CO2 from a single breath during vigorous exercise.2 This process entailed capturing exhaled gas using a system the size of a telephone booth consisting of a series of electro-mechanically controlled valves, which directed sequential portions of expired air into 6 small rubber bags. The collected CO2 in each bag was then analyzed multiple times to yield the average CO2, which was plotted over time, thus resulting in the first exhaled capnogram. By the 1970s, exhaled CO2 was monitored in ICUs using mass spectrometry systems that aspirated exhaled gas through long lengths of capillary tubing to a central monitoring location.3,4 Periodic measurements of the partial pressure of end-tidal CO2 (PETCO2) occurred on a timed schedule or in a sequential loop as the sampling cycle rotated between a number of monitored beds.4 With the introduction of the smaller infrared sensor, portable exhaled CO2 monitors came to the bedside in the 1980s.5 Miniature CO2 monitors that can fit into the palm of the hand and are capable of displaying an exhaled CO2 capnogram are currently available.6,7
The technological advancement of exhaled CO2 monitoring has coincided with the progression of use and importance in clinical practice. The significance of exhaled CO2 monitoring extends well beyond the very basic utilization of monitoring the adequacy of ventilation. Detection of exhaled CO2 has proven to be an invaluable mechanism for confirming tracheal intubation, recognizing accidental esophageal intubations, and other critical patient safety benefits. The patient protection enhancements provided by CO2 monitoring also include: the detection of invasive airway disconnection, dislodgement, or obstruction and monitoring for respiratory depression postoperatively, during procedural sedation, and during patient-controlled analgesia. Of critical significance is the use of PETCO2 to gauge the effectiveness of cardiopulmonary resuscitation (CPR), to predict outcome, and to guide the continuation of resuscitation efforts.8 Exhaled CO2 can also be described as the vital sign for ventilation and perfusion because the volume of CO2 excreted by the cardiorespiratory system is a sensitive indicator not only of ventilation efficiency but also of pulmonary perfusion and cardiac output. The volume of exhaled CO2 per minute (V̇CO2) can also be used to estimate the metabolic rate and nutritional requirements of the critically ill patient.9
The recognition and importance of monitoring exhaled CO2 in many areas of patient care has led to the expanding recommendation for its use by regulatory agencies and inclusion in the standards and practice guidelines of many professional organizations, such as the American Society for Anesthesia (ASA), the American Heart Association, the American Association for Respiratory Care, the Joint Commission, and the Centers for Medicare and Medicaid Services. Despite this heightened level of awareness and the regulatory and organizational guidance, exhaled CO2 monitoring is often underutilized.8,10–16
The reasons for this disparity between knowledge and practice may be due in part to the cost burden of acquiring the technology; the clinician time required for set-up, maintenance, and troubleshooting; and the lack of awareness of best practices for optimal utilization. Universally accepted standards of care mandating the use of CO2 monitoring in specific circumstances or patient categories yielding the highest potential to impact patient safety and influence outcomes are also lacking.
The purpose of this review is to increase the awareness and understanding of health-care providers of the importance of exhaled CO2 monitoring and its application in clinical practice. An appraisal of the different technologies will be followed by a review of the recognized indications for monitoring and an assessment of the potential for further advancements in exhaled CO2 monitoring.
Classification and Types of Exhaled CO2 Monitoring
Understanding available technologies and how they functions can provide important insights into their appropriate application, potential limitations, and problems associated with use. The following is a brief review of the classification and types of exhaled CO2 monitoring commonly utilized.
Colorimetric CO2 Detectors
Colorimetric CO2 detectors provide continuous qualitative and semi-quantitative exhaled CO2 monitoring. Colorimetric CO2 detectors are the simplest form of CO2 monitors. They are portable, disposable, inexpensive, single-use devices that can rapidly detect and confirm tracheal intubation and estimate the amount of CO2 in exhaled gas. Colorimetric detectors contain chemically treated material that is pH-sensitive and colorimetrically reflects CO2 concentrations in expired gas.17 Limitations of colorimetric CO2 detectors include false positive readings when the detection medium is contaminated with gastric acid or acidic solutions instilled through an endotracheal tube.17,18
Mainstream CO2 Monitoring
Mainstream CO2 monitoring devices utilize a small infrared sensor consisting of a sample cell or cuvette and an infrared optical bench placed within the gas flow pathway at the airway. This measurement location results in real-time CO2 values within the airway and a real-time graphical representation of the CO2 waveform plotted over time or by the exhaled volume. Disadvantages of mainstream CO2 monitoring include: cost of the sensor; potential for damage to the sensor and connecting cable during handling and patient monitoring; increased circuit mechanical dead space; potential for fouling with coughed up secretions and circuit condensate; and the additional weight of the sensor, airway adapter, and cable at the patient's airway connection. Also, many mainstream devices are limited to use with intubated patients only.19
Sidestream CO2 Monitoring
Sidestream CO2 monitoring devices aspirate a gas sample from a ventilator breathing circuit or other patient interface device through a length of small-bore tubing. Different interface designs for use on non-intubated patients incorporate nasal and oral sampling points to improve measurement accuracy. Several interface designs include the ability to simultaneously administer oxygen through a split channel device. Sidestream sampling devices utilize an infrared CO2 sensor in a monitor located away from the patient and can only be displayed in a time-based waveform. Time-based sidestream CO2 monitoring is the type more commonly used in the operating room setting, during non-intubated patient monitoring, and during CPR.
The sidestream sample method often requires use of a water trap and or specialized tubing that removes water vapor before analysis to prevent blockage and contamination of the sample tubing. Sample analysis located away from the patient results in a time delay often several seconds in duration before measured results are displayed. Since sidestream sampling requires a continuous aspirated sample flow rate from the ventilator circuit, interference with the measurement of ventilation parameters and the triggering mechanism can occur. Also, when sidestream systems are used intra-operatively with inhaled anesthetic agents, a scavenging system is required to prevent ambient contamination and health-care provider exposures.19
Capnometry Versus Capnography
Capnometry is performed by a capnometer. A capnometer is a monitor that measures CO2 concentrations in respired gases over time and displays numeric values for PETCO2, respiratory frequency, and sometimes the inspired CO2 concentration or partial pressure. Capnometers can be either mainstream or sidestream in design.20,21
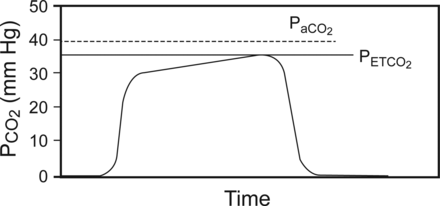
The terms capnometry and capnography are often used synonymously, but in contrast to capnometry, capnography is performed by a capnograph, a monitor that is functionally the same as a capnometer but incorporates the additional feature of displaying the CO2 waveform known as a capnogram (Fig. 1). Capnographs can also be mainstream or sidestream in design. The additional clinical monitoring value of capnography is the ability to visually interpret the waveform and morphology of the capnogram. The value of viewing the capnogram is synonymous with the revelation of knowing the heart rate and seeing the electrocardiogram. Interpretation of the different phases; the shape, area, and slope of the capnogram segments; and integration of the exhaled capnogram to exhaled tidal volume reveal clinically valuable information regarding ventilation and perfusion and cardiopulmonary physiology.20,21
Normal time-based capnogram showing the difference between the PaCO2 and partial pressure of end tidal CO2 (PETCO2), which is normally 2–5 mm Hg.
Indications for Exhaled CO2 Monitoring
Exhaled CO2 monitoring is essential and useful in clinical practice for an array of monitoring indications, including ventilation, circulation, metabolism, improving patient safety, and influencing and predicting outcomes of care. Following is a review of the indications for exhaled CO2 monitoring for in- and out-of-hospital monitoring of intubated and non-intubated adult and pediatric patients.
Monitoring Adequacy of Ventilation
Continuous monitoring of exhaled CO2 from the onset of intubation to extubation, whether by capnometry or capnography, is an accepted method to ensure adequate ventilation during mechanical ventilation in both adult and pediatric patients.14,22 CO2 monitoring in non-intubated adult,13,23,24 neonatal, and pediatric patients25–27 can also be effective. As a reflection of this potential benefit, the ASA has identified CO2 monitoring as a standard of care in anesthesia monitoring since 1986.28 In healthy patients during mechanical ventilation, PETCO2 closely approximates PaCO2 and is usually 2–5 mm Hg lower29–31 and therefore can be an effective index of the adequacy of ventilation (see Fig. 1).
However, it should be well understood by all clinicians that in diseased states, such as ARDS, COPD, and asthma, ventilation/perfusion (V̇/Q̇) mismatch in the lungs can cause the PaCO2 − PETCO2 difference to increase. An increased PaCO2 − PETCO2 difference can occur to varying degrees with increased physiologic dead space, shunt perfusion, low cardiac output, and conditions of low V̇/Q̇.
It has been suggested that the lack of agreement with PaCO2 in diseased lungs has negatively impacted the perception of value and the use of CO2 monitoring.8 Contrary to this misinterpretation, when a large PaCO2 − PETCO2 difference exists, PETCO2 can be an effective means of trending changes in PaCO2 and the efficiency of ventilation and changes in the physiologic dead space fraction (VD/VT).32 It is therefore important to recognize that the PaCO2 − PETCO2 difference can be an indication of the degree of lung injury and correlates with increased VD/VT.5,32,33
Furthermore, intra-operative monitoring of the PaCO2 − PETCO2 difference has been shown to predict mortality in trauma patients. Patients with a PaCO2 − PETCO2 difference >8–10 mm Hg had higher mortality rates both intra-operatively34–37 and following emergent surgeries.34,37
Other sometimes overlooked benefits of monitoring the PaCO2 − PETCO2 difference to assess the adequacy of ventilation include: the reduction of arterial blood gas utilization,38 which impacts unnecessary blood loss and costs; the identification and prevention of excessive ventilation and hyperventilation in neurotrauma patients39–43; and the affect that visual interpretation of the capnogram has had on enhancing monitoring during mechanical ventilation and airway management.44
Confirmation of Endotracheal Intubation
Perhaps one of the most significant impacts of exhaled CO2 monitoring is in its use as a definitive method for confirming endotracheal intubation and the reduction in harm associated with identifying the serious consequences of accidental esophageal intubation, death and anoxic brain injury. Use of a CO2 monitor in addition to direct visualization of the endotracheal tube passing through the vocal cords represent the accepted standards in clinical practice for confirming intubation in adult, pediatric, and neonatal patients.45–49
Capnography and capnometry compared with auscultation have been shown to be the most reliable methods to confirm endotracheal tube placement in the prehospital emergency setting.50 Several studies have demonstrated that when continuous PETCO2 monitoring is used, the sensitivity (true positive rate) and specificity (true negative rate) for confirming endotracheal intubation are both 100%.50–52 Colorimetric detectors were also found to be highly sensitive (88–98%) and 100% specific in confirming proper endotracheal intubation17,18 and useful in guiding and predicting successful resuscitation in non-cardiac arrest patients.17,18,53 Rates of unrecognized misplaced endotracheal tubes in out-of-hospital arrests as high as 23% have been reported when continuous PETCO2 monitoring was not used.52
Recognition of these important facts by the ASA,28,54 the American Association for Respiratory Care,55 the American Heart Association,56,57 Emergency Medical Services systems,42,58 the Intensive Care Society in the United Kingdom,42 the Royal College of Anesthetists, and the Association of Anesthetists of Great Britain and Ireland,16 has resulted in the inclusion of the use of CO2 monitoring during intubations in their respective standards of practice, clinical practice guidelines, and practice recommendations. This compelling body of evidence mandates the use of CO2 monitoring during intubation as a universal standard of care for confirming endotracheal intubation.11 Nevertheless, It should be well understood that during cardiac arrest, the accuracy for confirming proper endotracheal intubation using CO2 monitoring is diminished due the absence of cardiac output, pulmonary blood flow, and therefore exhaled CO2.17,18,50 In this situation, the absence of exhaled CO2 using any CO2 monitoring device may be mistakenly interpreted as an esophageal intubation.
Monitoring Respiratory Status
The use of exhaled CO2 to monitor respiratory status is another area of growing significance and interest. Exhaled CO2 offers an accurate and reliable means of measuring respiratory frequency. An elevated respiratory frequency is a sensitive and reasonably specific marker of respiratory dysfunction.59 A low respiratory frequency is an indicator of respiratory depression from opioid analgesia and can be detected early by exhaled CO2 monitoring.60
Opioid-induced respiratory depression in the post-anesthesia care period is a major concern emphasized by ASA-mandated standards,28 the Joint Commission Sentinel Event Alerts,61 and the Centers for Medicare and Medicaid Services Regulations and Interpretive Guidelines for Hospitals.62 Because oxygenation and ventilation are separate physiologic processes, pulse oximetry monitoring of oxygenation alone should not be considered a suitable monitor of ventilatory function.61–64 Oxygen saturation is frequently maintained, even at a low breathing frequency, and pulse oximetry alone often fails to detect respiratory depression, hypoventilation, and apnea episodes primarily when the patient is receiving supplemental oxygen.65 Therefore, the application of exhaled CO2 monitoring in the post-anesthesia setting, during procedural sedation, and when administering opiate analgesia can provide an important early warning of ventilatory compromise in adult and pediatric patients.65–69
A significant limitation to consider while using PETCO2 monitoring to detect respiratory depression is that there are 2 different types of drug-induced hypoventilation (Table 1). Type 1 or bradypneic hypoventilation is characterized by a decreased respiratory frequency, a slightly decreased tidal volume, and an increased PETCO2 and PaCO2. Type 2 or hypopneic hypoventilation, on the other hand, is characterized by a significant decrease in tidal volume, slightly decreased respiratory frequency, and a decreased or normal PETCO2, whereas the PaCO2 can be elevated.70
Characteristics of Bradypneic (Type 1) and Hypopneic (Type 2) Hypoventilation70
During hypopneic hypoventilation, the shallow breathing and smaller tidal volumes in proportion to the anatomical dead space cause decreased alveolar ventilation, increased dead space ventilation, increased PaCO2, and an increased PaCO2 − PETCO2 difference. This significant distinction between bradypneic and hypopneic hypoventilation is critical for clinicians to acknowledge and comprehend. Low or normal PETCO2 during hypopneic hypoventilation can be accompanied by significant respiratory depression and a markedly elevated PaCO2, which may go unrecognized if patient monitoring focuses on the value of PETCO2.
Another area of concern is the growing evidence that the use of patient-controlled analgesia (PCA) imposes significant risks to patients and potential liability exposure to health-care providers and hospitals. This is again reflected in position statements and guidelines from the ASA,71,72 the Centers for Medicare and Medicaid Services,62 the Joint Commission,73 the Anesthesia Patient Safety Foundation,74,75 and the Institute for Safe Medication Practices,64 which recommend continuous PETCO2 monitoring to guard against opioid-induced respiratory depression during PCA use. Of particular concern driving these recommendations are well-documented cases of “PCA by proxy” in which a family member or health-care provider, and not the patient, administers PCA doses. This has led to serious and fatal outcomes considered possibly preventable if PETCO2 monitoring were being used.73,76–78
Furthermore, the value of CO2 monitoring during procedural sedation8,26,28,71,79,80 and in the emergency department as an early indication of respiratory depression, especially when patients are receiving oxygen,65,81,82 to aid in the identification of the severity of illness and to improve patient safety13,70,83–85 is also increasingly being mandated for adult and pediatric patients.
Monitoring During CPR
It has been well substantiated that exhaled CO2 monitoring during CPR provides invaluable information regarding the correct placement of an advanced airway,28,50,52,56,57,86 the effectiveness of cardiac compressions,56,86–88 the return of spontaneous circulation,56,86,87,89,90 and the prediction of outcome and survival during cardiac arrest.86,87,91
The value of exhaled CO2 monitoring as a guide to the effectiveness of cardiac compressions was first demonstrated in 1978 from a series of 3 patients during CPR.91 Subsequent animal studies demonstrated that PETCO2 correlated with cardiac output and the adequacy of CPR and was useful as a guide to successful CPR and predicted the return of spontaneous circulation.92–95 These findings were confirmed in 4 studies of human subjects, which indicated that when PETCO2 was <10 mm Hg during CPR, patients did not survive96–99 (Fig. 2).
Mean end tidal carbon dioxide (PETCO2) differences between survivors and non-survivors after 20 min of advanced cardiac life support during out-of-hospital cardiac arrest. Subjects with PETCO2 levels of ≤10 mm Hg died before reaching the hospital. Data from Reference 98.
These data provide evidence to support a PETCO2 threshold target value >10 mm Hg during CPR. Additional investigations also demonstrated that changes in PETCO2 during resuscitation attempts predicted outcomes and return of spontaneous circulation,89,100 suggesting that PETCO2 could be useful in deciding when to terminate resuscitation efforts in adult and pediatric patients.101 When the value of PETCO2 at 20 min after the initiation of CPR was used as a screening test to predict return of spontaneous circulation, a threshold value of 14 mm Hg or less had a sensitivity, specificity, positive predictive value (accuracy of positive results), and negative predictive value (accuracy of negative results) of 100%.100
PETCO2 monitoring during CPR can be an effective tool to detect the effectiveness of external cardiac compression and the development of rescuer fatigue. Additionally, a sudden increase in continuously monitored PETCO2 during high quality CPR was associated with the return of spontaneous circulation89 (Fig. 3).
Time-compressed capnograms of end-tidal carbon dioxide (PETCO2) during cardiopulmonary resuscitation (CPR), showing ineffective cardiac compressions or rescuer fatigue (A) and return of spontaneous circulation (B). Courtesy Philips Healthcare.
This evidence supports the use of PETCO2 monitoring during CPR in both in- and out-of-hospital resuscitations. The use of quantitative waveform capnography interpretation has been incorporated into advanced cardiac life support training to confirm advance airway placement and to monitor resuscitation effectiveness since 2010.56 The union between evidence for clinical practice and technology has resulted in the availability of exhaled CO2 monitoring as a feature incorporated into new state-of-the-art defibrillators and transport monitors.
Volumetric Capnography
Volumetric capnography differs from standard capnography in that exhaled CO2 is plotted against the exhaled tidal volume. Analysis of the phases of the volumetric capnogram, the shape and curve morphology, and measurements based on calculations from the volumetric capnogram can reveal important information in regard to the efficiency of ventilation and perfusion, the physiologic dead space fraction, and the metabolic rate of the patient.102
Phases of the Volumetric Capnogram
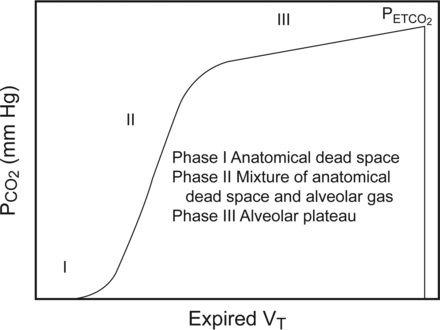
The 3 phases of the volumetric capnogram are synonymous with the phases of the time-based capnogram. Phase 1 characterizes emptying of the anatomic dead space (the conducting airways), where exhaled CO2 is near zero. Phase 2 represents a mixture of gas from the anatomic dead space and alveolar gas containing CO2. Phase 3 signifies emptying of alveolar air spaces (also known as the alveolar plateau), the end point of which is equal to the end-tidal CO2 (Fig. 4). An increase in slope of the rise of phase 3 signifies an increase in the number alveolar gas exchange units with varying degrees of V̇/Q̇ mismatch, such as during airway obstruction and elevated alveolar dead space.102
The 3 phases of the exhaled CO2 volumetric capnogram indicate the different stages of airway and alveolar gas emptying and terminate at the end-tidal carbon dioxide (PETCO2). Phase I = anatomical dead space. Phase II = mixture of anatomical dead space and alveolar gas. Phase III = alveolar plateau. From Reference 102.
Fowler Dead Space
Using the principles described by Fowler,103 the anatomical dead space volume can be identified and measured by computer analysis of the volumetric capnogram.104 The midpoint of phase 2 of the volumetric capnogram signifies the boundary between conducting airways and gas-exchange airways (respiratory bronchioles). When phase 2 is dissected by a vertical line originating from a line superimposed on the incline of phase 3, the intersection at the x axis such that the 2 areas on either side of the vertical line that are equal in area can be created. This point of intersection and equivalent areas is equal to the anatomic dead space volume on the volumetric capnogram (Fig. 5).
Anatomical dead space in male and female volunteers at rest was originally approximated to be 156 and 104 mL, respectively.103 Since the conducting airways are distensible, the anatomic dead space volume can increase with maximal voluntary inspiratory lung volume103 and by the application of positive pressure.105 The application of PEEP up to 18 cm H2O was shown to increase the anatomic dead space volume by an average of 5%.105 This increase in the anatomical dead space volume causes an incremental increase in the total physiologic dead space. The ability to measure anatomical dead space by volumetric capnography enables the components of physiologic dead space to be partitioned by single-breath analysis.
Single-Breath CO2 Analysis
The single-breath CO2 waveform analysis builds upon identification of the Fowler anatomical dead space and enables extraction of additional information regarding ventilation efficiency and further analysis and study of physiologic dead space.
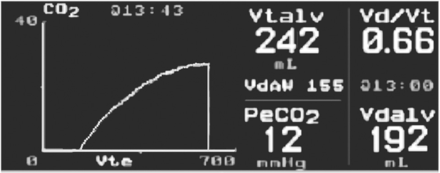
When the perpendicular line that identifies the anatomic dead space is drawn through the volumetric capnogram to the x axis, the point of intersection to this line from an additional line superimposed on phase 3 of the capnogram allows the component volumes in a exhaled tidal breath to be partitioned into anatomical VD, alveolar VD, and alveolar VT106,107 (Fig. 6).
Single-breath CO2 analysis further dissects the area of the volumetric capnogram and allows for the calculation of the alveolar dead space volume (VDalv), anatomic dead space volume (VDanat), alveolar tidal volume (VTalv), and the physiologic dead space volume (VDphys) in relation to PaCO2 and partial pressure of end-tidal CO2 (PETCO2). From Reference 106, with permission.
This dissection of the volumetric capnogram area allows further study of the various components and their relationships. For instance, alveolar VD and anatomical VD were both found to be indices of lung recruitment and overdistention,105 and the alveolar VD/alveolar VT ratio decreased or increased in parallel to a positive or negative response to PEEP with respect to oxygenation and shunt.108
Additionally, the classic sloping “shark's fin” shape and morphology of the time-based or volume-based volumetric capnogram can be a visual indication of V̇/Q̇ mismatch due to bronchospasm and airway obstruction14,109,110 (Fig. 7) and an elevated VD/VT in patients with ARDS (Fig. 8).
A “shark's fin” time-based capnogram waveform showing severe airway obstruction. From Reference 14.
A shark's fin volumetric capnogram of a patient diagnosed with ARDS with elevated dead-space fraction.
Measuring Physiologic Dead Space
The traditional technique for bedside measurement of VD/VT used the method of exhaled gas collection and analysis to measure the partial pressure of the mean expired CO2 (PĒCO2).111,112 Advancements in technology introduced the use of metabolic analyzers113,114 and volumetric capnography to measure the PĒCO2.115,116
The simplified bedside calculation of VD/VT uses the Enghoff modification of the Bohr equation.117 The Enghoff equation differs from the original Bohr equation by the substitution of PaCO2 for the partial pressure of mixed alveolar CO2 (PACO2).118 Since PACO2 was difficult to accurately measure or estimate at the bedside, the Enghoff equation became the standard method for many years in bedside clinical practice for calculation of VD/VT, where the Enghoff-Bohr equation is
 (1) and the original Bohr equation is
(1) and the original Bohr equation is
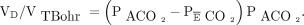 (2) In the past, PĒCO2 measurements using the gas collection bag method111,112 were cumbersome, time consuming, and error-prone and entailed collecting up to 30–60 L of expired gas for a mean CO2 sample measurement. Also, the gas collection bag method is no longer feasible due to the common presence of bias flow on current ventilators. The introduction of metabolic analyzers for the PĒCO2 measurement requires use of a correction for ventilator circuit compression volume116 and relies on the accurate measurement of the fraction of mean expired CO2 (FĒCO2), whereby
(2) In the past, PĒCO2 measurements using the gas collection bag method111,112 were cumbersome, time consuming, and error-prone and entailed collecting up to 30–60 L of expired gas for a mean CO2 sample measurement. Also, the gas collection bag method is no longer feasible due to the common presence of bias flow on current ventilators. The introduction of metabolic analyzers for the PĒCO2 measurement requires use of a correction for ventilator circuit compression volume116 and relies on the accurate measurement of the fraction of mean expired CO2 (FĒCO2), whereby
 (3) (PB represents barometric pressure, 47 is water vapor pressure at 37°C) The introduction of the volumetric CO2 monitor has simplified the bedside measurement by continuously measuring PĒCO2 and allowing intermittent input of PaCO2 for the calculation of VD/VT.115
(3) (PB represents barometric pressure, 47 is water vapor pressure at 37°C) The introduction of the volumetric CO2 monitor has simplified the bedside measurement by continuously measuring PĒCO2 and allowing intermittent input of PaCO2 for the calculation of VD/VT.115
PĒCO2 can also be derived from ventilator volumetric capnography measurements using V̇CO2 and expired minute ventilation (V̇E),116 whereby
 (4) By using Equation 3 to calculate PĒCO2, Equation 1 can then be used for the calculation of VD/VT.116
(4) By using Equation 3 to calculate PĒCO2, Equation 1 can then be used for the calculation of VD/VT.116
Clinical Usefulness of Physiologic Dead Space Measurements
Measuring VD/VT in the critically ill patient during mechanical ventilation is important for several reasons. The prognostic value of VD/VT has been linked to the severity of lung injury and the risk of death in patients with ARDS. Furthermore, VD/VT can be useful as an indicator of lung recruitment versus overdistention in patients with ARDS, may be helpful as a predictor of extubation success in adult and pediatric patients, and may be useful in assessing the severity and confirming the suspicion of pulmonary embolism.
Assessing the Severity of Lung Injury
VD/VT is known to be a marker of the type119 and severity of lung injury during ARDS in regard to the degree of hypoxemia,120 histopathological changes,120,121 V̇/Q̇ defects,122,123 intrapulmonary shunt,119,123,124 and the need for supportive rescue therapies.119,124 Monitoring serial changes in VD/VT over the course of critical illness in addition to other markers of disease severity is therefore warranted.
Predicting Survival in ARDS Patients
VD/VT has been shown to be predictive of the mortality risk in patients with ARDS in both the early and intermediate phases of the disease.125–131 During the first 2 d following an ARDS diagnosis, VD/VT was significantly higher among non-survivors131 (Fig. 9). Patients with VD/VT = 0.57 were found to have higher mortality, with a 45% increase in the odds of dying for every 0.05 increase in dead-space fraction.127 The mean dead-space fraction was also significantly higher in patients who died compared with those who survived (Fig. 10). Consequently, in the face of continually rising VD/VT during the course of ARDS as a sign of possible futility, it may be appropriate to use this information to help guide plan of care decisions during critical illness.
Dead space/tidal volume (VD/VT) in non-survivors compared with survivors was significantly higher on study day 1 and day 2 after diagnosis of ARDS. Data from Reference 131.
Dead-space fraction differences (mean ± SD) in ARDS survivors (0.63 ± 0.09) compared with non-survivors (0.54 ± 0.09) (P < .001). Data from Reference 127.
Indication of Lung Recruitment Versus Overdistention
VD/VT measurements in patients with ARDS have been found to be useful for titrating PEEP and optimizing cardiopulmonary function105,108,132 and may be useful as a tool to monitor lung recruitment versus overdistention.133–135
In one of the classic papers in critical care medicine published in 1975, Suter et al105 demonstrated the value of measuring VD/VT as a guide to optimizing gas exchange and pulmonary mechanics in patients with lung injury (Fig. 11). This early work has been repeatedly validated and therefore supports assessing and monitoring VD/VT to assist with management decisions in patients with ARDS.108,132,134,135
Changes in physiologic dead space (VDphys/VT), alveolar dead space (VDalv), and anatomic dead space (VDanat) fraction, in relation to shunt (VDshunt), total respiratory system compliance (Crs), cardiac output, and incremental changes in PEEP. From Reference 105, with permission.
Predicting Successful Liberation From Mechanical Ventilation
VD/VT assessments may also be used to predict extubation success in pediatric136 and adult patients.137,138 Values for VD/VT ≤ 0.50 and ≥ 0.65 in infants and children were found to predict extubation success or failure136 (Fig. 12), whereas in adult patients, the cutoff value for VD/VT = 0.58 offered the best sensitivity and specificity for predicting extubation failure.137 It has also been suggested that elevated VD/VT may be useful for predicting the need for noninvasive ventilation after extubation138 and therefore the potential for averting the need for re-intubation.
Graph plotting the differences of physiologic dead space to tidal volume (VD/VT) range against the percentage of subjects requiring additional ventilatory support after extubation. Data from Reference 136.
Diagnosis of Pulmonary Embolism
VD/VT, in addition to other clinical assessments and diagnostic tests, can be useful in excluding or diagnosing pulmonary embolism. VD/VT > 40% was found to be highly suggestive of pulmonary embolism and was comparable in terms of sensitivity and specificity with a lung perfusion scan.139 Assessment of the size of the capnogram waveform area was identified as a useful screening method for pulmonary embolism. When the capnogram area was low, signifying a larger PaCO2 to PETCO2 difference, the presence of pulmonary embolism was more likely.140 Conversely, when PETCO2 was normal (>36 mm Hg), the narrow PaCO2 − PETCO2 difference reliably excluded pulmonary embolism compared with contrast-enhanced helical computed tomography or ventilation/perfusion scan.141
The arterial to end-tidal alveolar dead space ratio ([PaCO2 − PETCO2]/PaCO2) cutoff value of 15% has also been shown to help exclude or confirm pulmonary embolism.141 When the alveolar VD/alveolar VT ratio is <20%, the probability of pulmonary embolism is low142–146 and was found to reduce the need for further diagnostic tests.148 On the other hand, an elevated alveolar VD/alveolar VT also predicts pulmonary embolism, the severity of the perfusion defect, and the degree of vascular occlusion.148
Bohr Versus Enghoff Approach to Physiologic Dead Space Measurements
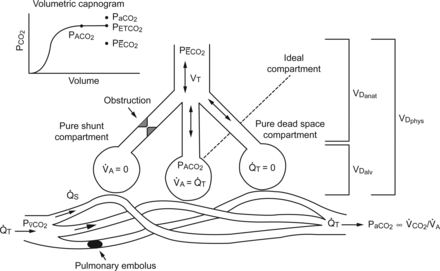
The Bohr dead space equation relies on the calculation or estimation of PACO2 from mixed alveolar gas and PĒCO2, which signifies the combined effect of the volume of anatomical VD, alveolar VD, and alveolar VT. PACO2 is affected by the dilution of CO2 from the alveolar side of the alveolar-capillary membrane from gas exchange units with low or no perfusion. PĒCO2 is determined by the volume of CO2 excreted from the lungs (V̇CO2) compared with the total V̇E (Equations 3 and 4). Bohr dead space is affected by areas of ventilation to perfusion inequality, such as during alveolar overdistention by excessive PEEP and/or VT, occlusion of the pulmonary vasculature, and low pulmonary perfusion secondary to hypovolemia or shock. Bohr dead space is recognized as true dead space, or the balance between effective and ineffective ventilation occurring in the lungs149 (Fig. 13).
The 3-compartment lung model described by Riley and Cournand151,152 represents gas exchange in the lung in regard to the matching of alveolar gas volume (V̇A) and perfusion (Q̇T), shunt (Q̇S), and dead space (VD). The ideal compartment represents areas of perfect alveolar gas volume to Q̇T matching. The pure shunt compartment represents areas of perfusion without ventilation. The pure dead-space compartment represents areas of ventilation with no perfusion. The sum of the regions of alveolar dead space (VDalv) and anatomic dead space (VDanat) equal the physiologic dead space (VDphys). The dead space fraction is equal to VDphys divided by tidal volume (VT). Also shown are PaCO2, the partial pressure of venous carbon dioxide (PvCO2), the relationship between PaCO2 and minute CO2 production (V̇CO2) and alveolar gas volume, the partial pressure of mixed alveolar carbon dioxide (PACO2), the partial pressure of end-tidal carbon dioxide (PETCO2), and the PĒCO2 in relation to the model and the volumetric capnogram. From Reference 116.
The Enghoff dead space equation, on the other hand, relies on the PaCO2 of arterial blood and is thus affected by global gas exchange inefficiency and the effects of shunt and venous admixture (Fig. 14). PaCO2 can be elevated from all sources of low ventilation to perfusion matching and shunt, such as atelectasis, pneumonia, COPD, and asthma. PaCO2 can also rise when an increase in metabolism and CO2 production is not accompanied by an increase in CO2 excretion. When V̇CO2 increases without a proportional rise in alveolar ventilation, production of CO2 exceeds the rate of excretion, and PaCO2 increases. Therefore, it is important to understand that the Enghoff equation overestimates VD/VT in the presence of shunt and low V̇/Q̇.
Graphical representation of physiologic dead space fraction determined by volumetric capnography, using the approaches of Bohr and Enghoff, which shows how use of the Enghoff equation can overestimate alveolar dead space (VDalv) (shaded areas) by substitution of the PaCO2 for the partial pressure of mixed alveolar carbon dioxide (PACO2), determined by identifying the midpoint of phase III of the expired volumetric capnogram.153,154 Also shown are the airway or anatomical dead space (VDanat; determined by the Fowler method identified at the midpoint of phase II of the expired volumetric capnogram),103 the partial pressure of end-tidal carbon dioxide (PETCO2), and the partial pressure of mean expired carbon dioxide (PĒCO2) in relation to the volumetric capnogram.150 From Reference 116.
The determination of PACO2 using volumetric capnography for the calculation of Bohr dead space has been demonstrated by mathematical modeling.152 and measured in an animal model of lung injury.153 PACO2 calculated from the phase 3 midpoint of the volume capnogram was compared with the PACO2 derived mathematically using the multiple-inert gas elimination technique. This demonstrated a close linear correlation between the 2 methods for PACO2 calculation (r = 0.99, P < .001) and Bohr dead space (r = 0.96, P < .001). The Bohr dead space and the mean PACO2 from volumetric capnography were similar to the multiple-inert gas elimination technique calculations, with a mean bias of 10 mL (95% CI −44 to 64 mL) and −0.10 mm Hg (95% CI −2.18 to 1.98 mm Hg), respectively.
The ability to measure PACO2 from the volumetric capnogram and therefore enabling the calculation of Bohr dead space has several significant implications. Measurement of Bohr dead space returns a more accurate reflection of effective and ineffective ventilation and perfusion in the lungs that is not impacted by the effects of shunt and low V̇/Q̇ on PaCO2 (Fig. 15).
Volumetric capnogram showing the relationship between PaCO2, the partial pressure of mixed alveolar carbon dioxide (PACO2), the partial pressure of end-tidal carbon dioxide (PETCO2), and the partial pressure of mean expired carbon dioxide (PĒCO2).
The effects of shunt on the measurement of VD/VT was investigated using a computer model simulation that controlled for variations of PACO2, alveolar VD, anatomical VD, pulmonary shunt, and V̇/Q̇ ratio distribution154 (Fig. 16) and in a lung lavage animal model using the midpoint of phase 3 to define PACO2155 (Fig. 17). Both experiments confirmed that increasing levels of shunt and low V̇/Q̇ markedly affects dead space values calculated by Enghoff's (Equation 1) but not Bohr's equation (Equation 2).
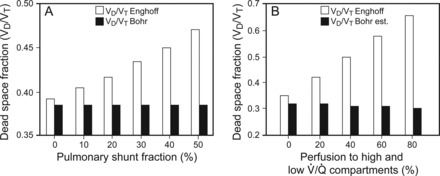
Dead-space (VD/VT) calculations using a mathematical computer model of the cardiorespiratory system. A: The effects of pulmonary shunt. B: The effects of pulmonary perfusion of high and low V̇/Q̇ compartments. VD/VTEngh is the dead-space calculated by using Enghoff's modification of the Bohr equation (Equation 1). VD/VTBohr is the dead space calculated by using the Bohr equation (Equation 2). VD/VTBohr est is the dead space calculated by using the Bohr estimate equation, which substitutes the partial pressure of end-tidal carbon dioxide (PETCO2) for the partial pressure of mixed alveolar carbon dioxide (PACO2) (Equation 5). Data from Reference 154.
Ratio of dead space to tidal volume (VD/VT) stratified according to the level of shunt in a surfactant-depleted swine model induced by repeated lung lavage. VD/VTEngh is calculated by Enghoff's modification of the Bohr equation (Equation 1). VD/VTBohr is calculated by Bohr's dead-space equation (Equation 2). Dashed trend lines show that the difference between VD/VTEngh and VD/VTBohr increases as pulmonary shunt increases. Data from Reference 155.
An estimate of the Bohr dead space can also be calculated by substituting PETCO2 for PACO2, whereby the Bohr estimate equation is
 (5) The comparison of VD/VTEngh, VD/VTBohr, and VD/VTBohr Est in regard to the area representing anatomical VD and alveolar VD on the volumetric capnogram is depicted in Figure 18. Example calculations based on the different equations in relation to the volumetric capnogram are shown in Figure 19.
(5) The comparison of VD/VTEngh, VD/VTBohr, and VD/VTBohr Est in regard to the area representing anatomical VD and alveolar VD on the volumetric capnogram is depicted in Figure 18. Example calculations based on the different equations in relation to the volumetric capnogram are shown in Figure 19.
Areas on the volumetric capnogram (in gray) representing the contribution of alveolar dead space to total physiologic dead space using PaCO2 (area a + b + c), the partial pressure of end tidal carbon dioxide (PETCO2) (area b + c), or the partial pressure of mixed alveolar carbon dioxide (PACO2) (area c) in relation to the partial pressure of mean expired carbon dioxide (PĒCO2), the anatomic dead space volume (VDanat), and the alveolar tidal volume (VTalv).
Example calculations based on the different equations in relation to the volumetric capnogram.
Figures 18 and 19 demonstrate that the Enghoff equation overestimates true VD/VT. Using the Bohr estimate equation also overestimates VD/VT, but this difference will always be closer to the true Bohr dead space than the Enghoff equation, whether VD/VT is normal or elevated. Although identifying PACO2 from the midpoint of phase 3 of the volumetric capnogram is technically achievable, using the Bohr estimate equation and substituting PETCO2 for PACO2 would simplify the calculation, especially when the midpoint of phase 3 is not clearly identifiable.
Since PĒCO2 can be calculated using Equation 4, and PETCO2 is available during volumetric CO2 monitoring, the continuous measurement of VD/VTBohr Est is technically feasible and would eliminate the requirement of periodic arterial blood gas sampling to measure PaCO2 for VD/VT calculation. The clinical value of constant monitoring and trending of VD/VTBohr Est during mechanical ventilation of the critically ill patient requires further investigation.
It has also been suggested that simultaneous assessment of Enghoff and Bohr dead space using volumetric capnography may be useful in regard to recognizing the effects of shunt and V̇/Q̇ inequality versus true dead space or wasted ventilation in critically ill patients with elevated VD/VT.149 Since many studies that identified VD/VT as a predictor of outcome, disease diagnosis, and disease severity used the Enghoff equation,125–131,136–138,141–148 the conclusions of these studies may need to be reexamined using this new approach.
Estimating Metabolic Rate and Resting Energy Expenditure
Estimation of metabolic rate can be accomplished by V̇CO2 measurements from volumetric capnography. The Weir equation is the accepted standard used during metabolic testing for calculating the nutritional support requirements in critically ill patients. Modifications to the Weir equation can be used to calculate resting energy expenditure (REE) by deriving a value for V̇O2 adjusted for a normal respiratory quotient (RQ) based on a measurement of V̇CO2. The REE by the Weir equation and the REE based on V̇CO2 (REE-CO2) can be calculated, where
 (6) and
(6) and
 (7) When the actual RQ is equal to 0.85, the REE-CO2 calculation results in an REE value equivalent to the value calculated by the standard Weir equation. The CCM Express metabolic analyzer (Medical Graphics Corporation, St Paul, Minnesota) uses Equation 7 to calculate REE-CO2 with an accuracy of approximately ±10% compared with the REE.156 The REE measured by the CCM Express using the standard Weir method was compared retrospectively with the REE-CO2 calculation in 67 adult ICU patients.157 The correlation coefficient r = 0.99 and the coefficient of determination r2 = 0.98, with bias and precision between measurements of −15 ± 126 cal/d. When the differences between REE and REE-CO2 were compared with the measured RQ, as the value for RQ moved toward 0.70, the percentage error (mean bias/mean REE for the range of RQ) became more positive, and as RQ moved toward 1.0, the percentage error became more negative. Furthermore, when RQ was normal in the range between 0.80 and 0.90, the average error was approximately ±5% (Fig. 20).157
(7) When the actual RQ is equal to 0.85, the REE-CO2 calculation results in an REE value equivalent to the value calculated by the standard Weir equation. The CCM Express metabolic analyzer (Medical Graphics Corporation, St Paul, Minnesota) uses Equation 7 to calculate REE-CO2 with an accuracy of approximately ±10% compared with the REE.156 The REE measured by the CCM Express using the standard Weir method was compared retrospectively with the REE-CO2 calculation in 67 adult ICU patients.157 The correlation coefficient r = 0.99 and the coefficient of determination r2 = 0.98, with bias and precision between measurements of −15 ± 126 cal/d. When the differences between REE and REE-CO2 were compared with the measured RQ, as the value for RQ moved toward 0.70, the percentage error (mean bias/mean REE for the range of RQ) became more positive, and as RQ moved toward 1.0, the percentage error became more negative. Furthermore, when RQ was normal in the range between 0.80 and 0.90, the average error was approximately ±5% (Fig. 20).157
Comparison of the differences between resting energy expenditure (REE) and resting energy expenditure based on V̇CO2 (REE-CO2) to the measured respiratory quotient (RQ). From Reference 157.
It should be noted that when using this approach, if the V̇CO2 measuring device has a substantial bias and precision difference from measurements by a metabolic analyzer, additional variance between REE and REE-CO2 will result. This difference in measurement accuracy can be compensated for by use of a measurement bias correction.
The REE-CO2 equation can be further simplified by solving the equation with any combination of V̇CO2 and V̇O2 that equals an RQ of 0.85, and dividing the calculated REE by the measured V̇CO2, a single factor can be derived for calculating REE-CO2, whereby
 (8) For example, when V̇CO2 = 221 mL/min and V̇O2 = 260 mL/min, RQ = 221/260 = 0.85, and the Weir equation returns a calculated REE of 1,810 kcal/d, whereby
(8) For example, when V̇CO2 = 221 mL/min and V̇O2 = 260 mL/min, RQ = 221/260 = 0.85, and the Weir equation returns a calculated REE of 1,810 kcal/d, whereby
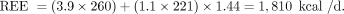 (9) REE-CO2 can be calculated as follows:
(9) REE-CO2 can be calculated as follows:
 (10)
(10)
 (11) and
(11) and
 (12) The caloric equivalence and carbon dioxide and oxygen can also be used to indirectly calculate REE from measurements of V̇CO2 (Table 2).158–160 When the RQ = 0.90, the CO2 caloric equivalent factor equals 5.52 kcal/L, where
(12) The caloric equivalence and carbon dioxide and oxygen can also be used to indirectly calculate REE from measurements of V̇CO2 (Table 2).158–160 When the RQ = 0.90, the CO2 caloric equivalent factor equals 5.52 kcal/L, where
 (13) This technique was compared with the Harris-Benedict calculation and the Weir equation. The Harris-Benedict equation significantly underestimated REE, but there was no significant difference between the Weir and REE-CO2 equivalent calculation158 (Table 3). Because of the technical difficulty of measuring V̇O2, the REE-CO2 equivalent calculation is simpler and should be the preferred method, especially when the FIO2 is >0.60.159
(13) This technique was compared with the Harris-Benedict calculation and the Weir equation. The Harris-Benedict equation significantly underestimated REE, but there was no significant difference between the Weir and REE-CO2 equivalent calculation158 (Table 3). Because of the technical difficulty of measuring V̇O2, the REE-CO2 equivalent calculation is simpler and should be the preferred method, especially when the FIO2 is >0.60.159
Comparison of 4 Methods to Determine Basal Energy Expenditure or Resting Energy Expenditure158
The ability to perform metabolic measurements using V̇CO2 from volumetric capnography has several important implications. V̇CO2 measurement capabilities are increasingly more available on standalone monitors and ventilators. This makes estimates of REE more readily accessible when metabolic analyzers are not available. When V̇CO2 is being monitored either continuously or intermittently during mechanical ventilation, if the REE-CO2 calculation is incorporated into the ventilator, estimates of the metabolic rate and nutritional requirements could be available in real time and trended over the course of critical illness.9
Additionally, since FIO2 does not affect the accuracy of the REE-CO2 calculation, FIO2 > 0.60 may no longer be a limitation of measuring a reasonable estimate of REE. The implications of using the REE-CO2 calculations are significant in that even the most severe critically ill patients receiving mechanical ventilation receiving 100% oxygen and high levels of PEEP can have REE estimates performed to manage their complex nutritional needs.
Since volumetric capnography and V̇CO2 monitoring are becoming more readily available and are less costly than traditional metabolic testing, their use should be considered for incorporation into a standard nutrition assessment and treatment process. Further validation studies and measurements of outcome are needed to verify the impact of these alternative methods of indirect calorimetry.
Summary
Monitoring exhaled CO2 goes beyond the basic use of evaluating and assessing ventilation. The uses of exhaled CO2 monitoring include improvements in patient safety, utilization in assessing care and evaluating interventions, identifying abnormal physiology, quantifying the severity of illness, and predicting outcomes. In recognition of these significant benefits, the importance of exhaled CO2 monitoring has been increasingly emphasized by practice standards, guidelines, and advisory statements from professional organizations and regulatory agencies.
Future advancements in volumetric capnography will include the capability to continuously estimate Bohr dead space and metabolic rate during mechanical ventilation. These advancements are currently at the verge of technical feasibility, and device manufacturers will soon bring these important enhancements in patient monitoring into clinical practice at the bedside. The critical impact and implications of these valuable monitoring methods utilizing exhaled CO2 will be important for all health-care providers to comprehend and utilize.
Footnotes
- Correspondence: Mark S Siobal RRT-ACCS FAARC. E-mail: siobalm{at}smccd.edu.
Mr Siobal presented a version of this paper at the 31st Annual New Horizons Symposium: Monitoring of the AARC Congress 2015, held November 7–10, 2015 in Tampa, Florida.
- Copyright © 2016 by Daedalus Enterprises
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.
- 13.↵
- 14.↵
- 15.
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.
- 36.
- 37.↵
- 38.↵
- 39.↵
- 40.
- 41.
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.
- 47.
- 48.
- 49.↵
- 50.↵
- 51.
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.
- 64.↵
- 65.↵
- 66.
- 67.
- 68.
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.↵
- 92.↵
- 93.
- 94.
- 95.↵
- 96.↵
- 97.
- 98.↵
- 99.↵
- 100.↵
- 101.↵
- 102.↵
- 103.↵
- 104.↵
- 105.↵
- 106.↵
- 107.↵
- 108.↵
- 109.↵
- 110.↵
- 111.↵
- 112.↵
- 113.↵
- 114.↵
- 115.↵
- 116.↵
- 117.↵
- 118.↵
- 119.↵
- 120.↵
- 121.↵
- 122.↵
- 123.↵
- 124.↵
- 125.↵
- 126.
- 127.↵
- 128.
- 129.
- 130.
- 131.↵
- 132.↵
- 133.↵
- 134.↵
- 135.↵
- 136.↵
- 137.↵
- 138.↵
- 139.↵
- 140.↵
- 141.↵
- 142.↵
- 143.
- 144.
- 145.
- 146.↵
- 147.
- 148.↵
- 149.↵
- 150.↵
- 151.↵
- 152.↵
- 153.↵
- 154.↵
- 155.↵
- 156.↵
- 157.↵
- 158.↵
- 159.↵
- 160.↵