Abstract
BACKGROUND: Infrequent serious complications of convex-probe endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) have been reported. The aim of this study was to assess serious complications related to convex-probe EBUS-TBNA and to determine the complication rate in a large group of subjects.
METHODS: In this retrospective study, a 15-item questionnaire on features of cases with EBUS-TBNA complications was sent to experienced bronchoscopists performing convex-probe EBUS-TBNA at 3 pulmonary centers. The medical records were then reviewed by these bronchoscopists to complete the questionnaire. Hemorrhage responsive to topical treatment, temporary laryngospasm/bronchospasm, transient oxygen desaturation, and fever lasting <24 h were excluded. Only complications requiring further treatment/intervention were considered serious. The rate of serious complications was calculated from the obtained data.
RESULTS: In a total of 3,123 cases within a 5-y period, EBUS-TBNA was performed for staging lung cancer in 15.8%, diagnosis in 67.5%, and diagnosis and staging in 16.3%. Of the 3,123, 11.6% had parenchymal lesions adjacent to major airways. EBUS-TBNA was performed 11,753 times (3.76/case) at 6,115 lymph node stations and lesions (1.92/station or lesion). Five serious complications were recorded (0.16%): fever lasting >24 h, infection of bronchogenic cyst, mediastinal abscess, pericarditis, and pneumomediastinitis with empyema, each in one case. Four complications occurred in cases diagnosed with benign disease by EBUS-TBNA. All complications were treated with broad-spectrum antibiotics. Four subjects were hospitalized for 21.7 ± 20.7 d.
CONCLUSIONS: Convex-probe EBUS-TBNA is a safe method in general. However, serious complications, including infections, can be encountered rarely. All precautions should be taken for complications before and during the procedure.
Introduction
Convex-probe endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is a minimally invasive method for investigating the mediastinum, for staging of non-small-cell lung cancer, and for diagnosing mediastinal lesions accessible via the major airway. It is increasingly used because of its safety, simplicity, high diagnostic yield, and ability to diagnose both benign and malignant conditions. The cumulative sensitivity and specificity of EBUS-TBNA in the lymph node staging of lung cancer is 88–93% and 100%, respectively.1
It is a noninvasive technique with minor complications of agitation, cough, and bleeding at the puncture site.2,3 Varela-Lema et al2 published a systematic review and noted no important complications related to >1,500 EBUS-TBNA procedures. A meta-analysis on EBUS-TBNA reported a complication rate of 2 in 1,299 cases (0.15%), with only 1 case showing pneumothorax requiring drainage as a major complication.4
EBUS-TBNA-related complications do occur, although the complication rate is low. Mediastinal abscess, empyema, mediastinal emphysema, pericarditis, sepsis, needle breakage, intramural hematoma of the pulmonary artery, hemopneumomediastinum, and lung abscess are major complications that have been reported in recent years, mostly as case reports.2,5–9 Systematic reviews report only 1 incidence of morbidity in a subject with pneumothorax after the procedure (0.07% morbidity).4,10 A few deaths following EBUS-TBNA have also been reported related to massive hemorrhages, stroke, and sepsis.11 Death due to cerebral infarction was reported in 1 case (1.3%) in the study of Asano et al.12 Navani et al13 also reported a death due to sepsis after EBUS-TBNA. These reports have raised a debate on the safety of endosonography.14 The complication rates were very low in the initial studies. However, these studies included EBUS-TBNAs performed by expert bronchoscopists, and study populations were relatively small for a reliable analysis of complications. Thus, these results may be inconsistent with those of routine clinical practice.12,15 At present, the data on the true incidence of EBUS-TBNA-related complications in everyday clinical practice are scarce. The frequency of these complications is likely to increase as the technique is more widely adopted and used.
Although there are many case reports on the complications of EBUS-TBNA in the related literature, to date, there have been few investigations on the types and rates of complications related to EBUS-TBNA. The aim of the present study was to assess type and rate of serious complications related to EBUS-TBNA.
QUICK LOOK
Current knowledge
Convex-probe endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is a minimally invasive method for investigating the mediastinum, for staging of non-small-cell lung cancer, and for diagnosing mediastinal lesions. It is increasingly used due to safety, simplicity, high diagnostic yield, and the ability to diagnose both benign and malignant conditions.
What this paper contributes to our knowledge
Convex-probe EBUS-TBNA was safe and effective in staging and diagnosis of lung cancer in >3,000 procedures. Infectious complications were rare, occurring in <0.2% of the procedures.
Methods
Study Design
This retrospective multi-center study was carried out between October 2008 and January 2014 in the pulmonary departments of 3 tertiary referral hospitals in Turkey. Convex-probe EBUS-TBNA was performed for staging or diagnostic purposes. A questionnaire with 15 questions was used and answered from subjects' medical records, including questions on age and sex of cases, the number of cases undergoing EBUS-TBNA, indications of EBUS-TBNA, anesthetic technique, total number of lymph node stations or lesions sampled by EBUS-TBNA, number of cases with non-lymph node lesions sampled by EBUS-TBNA, total number of needle passes, number of cases with serious complications, details of complications from subject background and charts, previous treatments related to underlying diseases, location of lesion or lymph node station, short axis diameter of lymph node or other lesion, final diagnosis, and treatment details of the complication (Table 1). The study protocol was approved by the local ethics committee, and written informed consent for EBUS-TBNA was obtained from all included subjects before the procedure.
Questionnaire for Convex-Probe Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration Complications
Serious Complications
Lidocaine intoxication requiring special intervention, respiratory failure requiring interventions other than oxygen administration, pneumonia, mediastinitis, pericarditis, other infectious complications, fever lasting longer than 24 h, pneumothorax requiring bed rest or thoracic drainage, prolonged bronchospasm, and hemorrhage not responsive to application of topical adrenaline and/or cold saline and requiring further intervention were considered as serious complications. However, hemorrhage other than described above; temporary laryngospasm, bronchospasm, and desaturations during the procedure; and fever lasting <24 h after EBUS-TBNA were not included in the serious complications.
All of the subjects were seen at least once within 3 d after the EBUS procedure to receive the pathology reports, and they were interviewed routinely for the possibility of complications. All subjects were kept in the hospital for at least 4 h after EBUS-TBNA. After this period, in the presence of symptoms or complaints, a chest x-ray was obtained. After EBUS-TBNA, a chest x-ray was obtained in all cases with peribronchial lesions.
Convex-Probe EBUS-TBNA
Convex-probe EBUS-TBNA from hilar and mediastinal lymph nodes was performed as indicated after physical examination, routine biochemical analysis, pulmonary function tests, and thoracic computed tomography and/or positron emission tomography-integrated computed tomography were performed. EBUS-TBNA was performed in all cases as an out-patient procedure in dedicated bronchoscopy suits using convex-probe bronchoscopes (Olympus 7.5 MHz, BF-UC160F and BF-UC160F-OL8, Olympus Optical, Tokyo Japan) with an EU C2000 processor (Olympus Optical). The scope was inserted through the oral route, in supine position and under local anesthesia with lidocaine and conscious sedation with intravenous midazolam, midazolam + opioid, midazolam + propofol, or ketamine + propofol. A 22-gauge NA-201SX-4022-C needle (Olympus Optical) was used for the procedure. Short and long axis diameters of a lymph node or other lesion, station of the lymph node, and number of needle passes per subject and per lymph node or other lesion were recorded for each subject.
Convex-probe EBUS-TBNA was performed at 3 different medical centers by 6 pulmonologists. In all centers, all cases were included in the study, from the first case when EBUS-TBNA was initiated in each center to the end of the study period.
Results
Of the 3,123 cases included in the study, 861 (27.6%) were females, and 2,262 (72.4%) were males, with a mean age of 54.8 ± 10.1 (minimum 16; maximum 83) y. EBUS-TBNA was performed for staging in 493 cases (15.8%), for diagnosis in 2,109 cases (67.5%), and for diagnosis and staging in 521 cases (16.3%). Three hundred sixty-two (11.6%) of the lesions sampled by EBUS-TBNA were peribronchial or intraparenchymal lesions adjacent to major airways, whereas 2,761 (88.4%) were hilar or mediastinal lymphadenopathies or mediastinal lesions.
EBUS-TBNA was performed at 6,115 lymph node stations or lesions (1.96/case). There were 11,753 needle passes in 3,123 cases (3.76/case and 1.92/station or lesion). Malignancy was the final diagnosis in 1,383 cases (44.3%), whereas it was benign disease or reactive adenitis in 1,740 cases (55.7%).
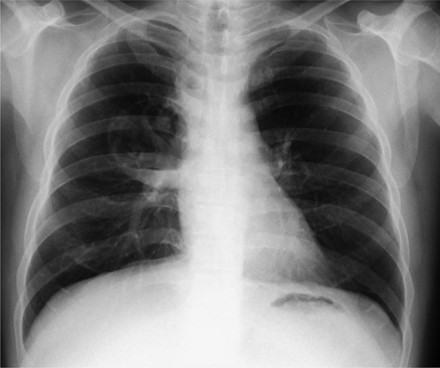
In total, 5 (0.16%) serious complications were recorded; there were no deaths. The serious complications were fever lasting longer than 24 h, infection of bronchogenic cyst, mediastinal abscess (Figs. 1⇓–3), pericarditis, and pneumomediastinitis with empyema. Each complication developed in a different subject. The characteristics of these subjects are given in Table 2. All subjects were treated with broad-spectrum antibiotics. Four subjects were hospitalized for 21.7 ± 20.7 d (7–51 d), and all subjects were discharged with improvement. We detected pneumomediastinum accompanying empyema in 1 case as a noninfectious serious complication (0.032%).
Chest x-ray of the subject with mediastinal abscess.
Mediastinal section of thoracic computed tomogram of subject with mediastinal abscess.
Parenchymal section of thoracic computed tomogram of subject with mediastinal abscess.
Features of Cases With Serious Complications
Only 1 (1 of 1,383, 0.072%) of these 5 cases was diagnosed with lung cancer metastasis in the lymph node sampled by EBUS-TBNA. Benign diagnoses (4 of 1,740, 0.22%) were obtained in all other cases. A total of 10 bronchogenic cysts or cystic lesions were detected in the study population, and in 2 of these (2 of 10, 20%) serious complications developed.
Discussion
This is the first study in Turkey regarding complications of EBUS-TBNA. To the best of our knowledge, there are 2 systematic reviews and 2 Japanese survey studies in the literature describing the serious complications associated with EBUS-TBNA.2,4,12,14 The serious complication rate of EBUS-TBNA was determined to be 0.16% in the present study.
The serious complication rate of endoscopic ultrasonography and convex-probe EBUS-TBNA was reported as 0.14% in the systematic review of von Bartheld et al.14 They reported 5 serious adverse events, consisting of 2 infectious complications, 2 pneumothorax, and 1 airway edema and hypoxemia, in 9,119 EBUS-performed cases (0.05%). There is no mortality reported either in this review or in our study. A survey study conducted in Japan revealed a complication rate of 0.46% after EBUS-TBNA of hilar and/or mediastinal lymph node lesions.15 In another Japanese survey study reported later, it was stated that 50 detected complications (0.68%) were hemorrhage, and 14 (0.19%) were infectious complications in 7,345 cases undergoing convex-probe EBUS-TBNA. However, there was not a specific definition for serious complications in this study. It was also reported that life-threatening complications were observed in 4 cases, and a longer hospital stay was required in 14 cases. In this study, death was also reported in 1 case because of cerebral infarction (mortality rate of 0.01).12 Eapen et al16 reported a complication rate of 1.44% in their prospective study. A meta-analysis of EBUS-TBNA showed a complication rate of 2 in 1,299 cases (0.15%).4 In this meta-analysis, only 1 case with pneumothorax requiring drainage was reported as a serious complication. However, the studies evaluated in this meta-analysis were performed by expert operators.
With the recent widespread use of EBUS-TBNA, there have been an increasing number of reports on complications associated with its use.5–9,12,14 The incidences of complications may vary depending on several factors. First, follow-up of patients to detect possible complications is not often a routine procedure, and lack of documentation is also an important factor, especially for minor complications. Second, the definition of complications and their severity can affect the rates and descriptions of the complications.
Considering the investigations and case reports reported in the literature, infectious complications constitute a significant proportion of serious complications (5–8,12,17–19). In our series, all 5 serious complications were infections. Pneumomediastinum accompanying empyema was also diagnosed in 1 case.
Prolonged hospitalization was required in 4 of 5 subjects with serious complications in the current study. Similarly, Asano et al12 reported prolonged hospitalization in 14 (15.6%) of 90 cases with complications of EBUS-TBNA of a total of 7,345 cases.
It has been suggested that infectious complications occurring after EBUS-TBNA are due to seeding at the puncture site rather than bacteremia after hematogenic spread.5,12,14 In our study, Streptococcus pneumoniae and Streptococcus constellatus growths were detected on blood culture of 2 complicated cases. von Bartheld et al14 reported that cases with mediastinal cyst and sarcoidosis had more risk for infectious complications. In a study on the diagnostic methods for mediastinal benign cysts, Wildi et al17 determined that mediastinitis developed in 1 of 4 cases with mediastinal cystic lesions punctured by EBUS-TBNA, whereas there were no complications reported in the remaining 3 cases receiving prophylactic antibiotics. The infection at the puncture site develops with cross-contamination of floral bacteria of the oral cavity and nasopharynx.5,9,12 S. constellatus growing on blood culture of one of our cases is also within the floral bacteria of oral cavity and nasopharynx. In our study, EBUS-TBNA was performed on mediastinal cystic lesions in 2 (40%) of the 5 cases with infectious complications, and there were a total of 10 cases with cystic lesions.
von Bartheld et al14 indicated that endoscopic ultrasound and EBUS are safe techniques for an intrathoracic investigation of the mediastinum in subjects with suspected lung cancer. They reported that only 2 cases of severe infectious complications have been described in lung cancer subjects. One of them was due to technical problems, and the other was due to necrotic lymph nodes.14 In our study, the serious complication rate was 0.072% among the cases diagnosed as malignant. Previous studies have suggested that the presence of necrosis in the mediastinal lymph nodes of subjects with malignancy could be a risk factor for developing mediastinal infections due to avascularity of the lesion.14,20 We did not detect necrosis by computed tomography or EBUS in any of the lymph nodes/lesions in the complicated malignant cases of this study.
Haas9 reported 2 infectious complications of EBUS-TBNA. He pointed out that development of post-procedure infectious complications appeared to be caused by full-needle extension during EBUS-TBNA. For pericarditis occurring in one subject, he commented that full-needle extension must have allowed the needle tip to enter the pericardial space and caused pericardial contamination because the needle tip can be difficult to visualize in all planes when fully extended.9 Our case with pericarditis had a 22.5-mm cystic paratracheal lesion. No systemic bacteremia could be shown in this case, similarly to those in the study of Haas.9
Hata et al21 reported 2 cases of tuberculosis after EBUS-TBNA. One of them was complicated with the development of intrabronchial polypoid lesions at the puncture sites nearly 2 months after the EBUS-TBNA procedure at hilar and subcarinal lymph nodes. The other one had positive sputum smear for acid-fast bacilli after the EBUS-TBNA, whereas she was sputum-negative before the procedure. In the present study, 1 of the 5 cases with serious complications of EBUS-TBNA developed mediastinal abscess and was also diagnosed with tuberculous lymphadenitis by EBUS-TBNA. Gupta et al22 reported a patient with tuberculous lymphadenitis in whom an endobronchial polyp developed following EBUS-TBNA. We saw neither endobronchial lesion nor sputum smear positivity after EBUS-TBNA. The subject in our study fully recovered with antibiotic treatment. Hata et al21 stated that there is a possibility of aggravation caused by puncturing the bronchial mucosa. Excessive puncturing should therefore be avoided in patients with suspected tuberculous lymphadenitis.21 Although non-infectious serious complications, such as needle breakage, hemorrhage, intramural hematoma of the pulmonary artery, and hemopneumomediastinum, have also been reported in the related literature, we only encountered infectious complications.
The limitations of this study are its retrospective design and lack of long-term follow-up of all cases for the assessment of complications after EBUS-TBNA. It is possible that both of these limitations could lead to underestimation of the complication rate yet, but it is unlikely that the overall rate would be significantly different.
Conclusions
In conclusion, convex-probe EBUS-TBNA is a safe method in general but infrequently can cause serious infections. All precautions should be taken for complications before and during the procedure. After evaluation of the results of our study and the data obtained from the literature, we recommend to avoid performing EBUS-TBNA on mediastinal cystic lesions or to use a prophylactic antibiotic if it is necessary to perform EBUS-TBNA.
Footnotes
- Correspondence: Sevda Şener Cömert MD, Pembe Köşk sok. Emek apt. No:16 D:14 Merdivenköy Kadıköy-İstanbul 35732, Turkey. E-mail: sevdasener2{at}yahoo.com.
Dr Çağlayan presented a version of this paper at the European Respiratory Society International Congress 2014, held September 6–10, 2014, in Munich, Germany.
The authors have disclosed no conflicts of interest.
- Copyright © 2016 by Daedalus Enterprises