Abstract
BACKGROUND: Non-ventilator ICU-acquired pneumonia after cardiothoracic surgery is challenging to diagnose, and little is known about its impact on patient outcomes. Here, our primary objective was to compare the sensitivity and specificity of cultures of 2 types of fiberoptic bronchoscopy (FOB) specimens: endotracheal aspirates (FOB-EA) and bronchoalveolar lavage fluid (FOB-BAL). The secondary objectives were to evaluate the sensitivity and specificity of spontaneous sputum cultures and of the modified Clinical Pulmonary Infection Score (CPIS) and to describe patient outcomes.
METHODS: We conducted a prospective observational study of consecutive cardiothoracic surgery subjects with suspected non-ventilator ICU-acquired pneumonia. Using FOB-BAL cultures ≥104 cfu/mL as the reference standard, we evaluated the accuracy of FOB-EA ≥105 cfu/mL and spontaneous sputum ≥107 cfu/mL. On the day of FOB, we determined the modified CPIS. Mortality and antibiotic treatments were recorded.
RESULTS: Of 105 subjects, 57 (54.3%) received a diagnosis of non-ventilator ICU-acquired pneumonia. FOB-EA cultures had 82% (95% CI 69–91%) sensitivity and 100% (95% CI 89–100%) specificity and were significantly less sensitive than FOB-BAL cultures (P < .004). Spontaneous sputum was obtained from one-third of subjects. Spontaneous sputum cultures had 82% (95% CI 56–95%) sensitivity and 94% (95% CI 68–100%) specificity and were non-significantly less sensitive than FOB-BAL (P = .061). A modified CPIS >6 had 42% (95% CI 29–56%) sensitivity and 87% (95% CI 74–95%) specificity for non-ventilator ICU-acquired pneumonia. Antibiotic therapy was stopped in all subjects without non-ventilator ICU-acquired pneumonia, after 1.6 ± 1.2 d, without deleterious effects.
CONCLUSIONS: The modified CPIS has low diagnostic accuracy for non-ventilator ICU-acquired pneumonia. FOB-EA cultures perform less well than do FOB-BAL cultures for diagnosing non-ventilator ICU-acquired pneumonia. Spontaneous sputum is valuable when FOB cannot be performed but could be obtained in only a minority of subjects. When cultures are negative, antibiotic discontinuation is safe.
- postoperative pneumonia
- cardiothoracic surgery
- fiberoptic bronchoscopy
- endotracheal aspirates
- spontaneous sputum
- bronchoalveolar lavage
- sensitivity
- specificity
- antibiotics
- mortality
Introduction
Postoperative pneumonia is among the most common complications after cardiothoracic surgery. This specific form of hospital-acquired pneumonia is managed on the surgical ward or, when severe, in the ICU.1 Risk factors for specific pathogens are particularly prevalent in ICU patients.2 Postoperative pneumonia may be acquired in the ICU, in patients receiving mechanical ventilation1,3 (ventilator-associated pneumonia [VAP]) or in non-ventilated patients1,2 (non-ventilator ICU-acquired pneumonia). Because nearly 90% of episodes of hospital-acquired pneumonia occur during mechanical ventilation,1 non-ventilator ICU-acquired pneumonia has received little research attention. The diagnosis and treatment of non-ventilator ICU-acquired pneumonia remain challenging.2,4,5 Few studies have assessed the accuracy of clinical diagnostic methods. In particular, the Clinical Pulmonary Infection Score (CPIS)6 based on clinical variables has been suggested for the diagnosis and management of VAP6,7 but has not been evaluated in non-ventilator ICU-acquired pneumonia. Studies in VAP or community-acquired pneumonia1,3,8 have established that fiberoptic bronchoscopy (FOB) with bronchoalveolar lavage (FOB-BAL) provides reliable pulmonary specimens for microscopic smear examination and cultures in subjects with clinically suspected pneumonia. This procedure may be equally useful in non-ventilator ICU-acquired pneumonia. Obtaining endotracheal aspirates during FOB (FOB-EA) might be an easier and faster technique than FOB-BAL for diagnosing non-ventilator ICU-acquired pneumonia but has produced conflicting results for the diagnosis of VAP or community-acquired pneumonia.1,3,8,9–11 Importantly, FOB has been reported to induce respiratory status deterioration in a substantial proportion of subjects.12 On the other hand, culturing spontaneous sputum is a noninvasive test but varies widely in its yield, in large part due to the quality of the sampling process.8,13,14 The absence of a reliable diagnostic tool may result in patients with suspected non-ventilator ICU-acquired pneumonia receiving unnecessary antibiotic therapy or, on the contrary, failing to receive antibiotics they need.
The primary objective of this prospective observational study was to compare the diagnostic accuracy of bacterial cultures of FOB-EA, using FOB-BAL as the reference standard,7 in subjects with clinically suspected non-ventilator ICU-acquired pneumonia after cardiothoracic surgery. Our secondary objectives were to evaluate the diagnostic accuracy of spontaneous sputum and the modified CPIS and to describe the outcomes of subjects with and without a diagnosis of non-ventilator ICU-acquired pneumonia.
QUICK LOOK
Current knowledge
Non-ventilator ICU-acquired pneumonia is a specific form of hospital-acquired pneumonia. Diagnostic methods and management of such pneumonia have not been evaluated.
What this paper contributes to our knowledge
Fiberoptic bronchoscopy with bronchoalveolar lavage was the best investigation for diagnosing non-ventilator ICU-acquired pneumonia. Spontaneous sputum expectoration is valuable when fiberoptic bronchoscopy cannot be performed but can be obtained only in a minority of patients. Subjects without non-ventilator ICU-acquired pneumonia based on negative culture results experienced favorable outcomes without prolonged antibiotic therapy.
METHODS
Subject Selection
From December 1, 2011, to January 31, 2013, we prospectively included consecutive subjects in a 19-bed cardiothoracic surgical ICU who met our selection criteria. The study was approved by the Comité de Protection des Personnes Ile-de-France VII (No. SC12–007) and considered part of standard care. Written informed consent was waived, but all subjects or family members were informed about the study.
Subjects were included if they met the following criteria: ICU admission after cardiothoracic surgery; spontaneous breathing; age ≥18 y; and clinically suspected non-ventilator ICU-acquired pneumonia based on CPIS variables.6 In subjects receiving oxygen via a nasal cannula or simple face mask, FIO2 was assumed to increase by 3% for each liter of oxygen. In subjects given oxygen via a non-rebreathing mask with a reservoir, FIO2 was assumed to be 80%.
Exclusion criteria were tracheostomy, bradypnea, hemodynamic instability, nausea or vomiting, confusion or delirium, and acute respiratory failure precluding FOB because of a high risk of endotracheal intubation. None of the subjects underwent lung or heart-lung transplantation.
Data Collection
The following data were recorded prospectively: age, sex, body mass index, history of diabetes or chronic obstructive pulmonary disease, ongoing corticosteroid therapy, and type of surgery. Severity of the acute illness was evaluated using the Simplified Acute Physiology Score II and Sequential Organ Failure Assessment score.
On the day of bronchoscopy (day 0), we recorded the highest temperature (°C), PaO2/FIO2, leukocyte count, concomitant bacteremia, prior antimicrobial therapy, initiation and duration of antimicrobial therapy, and use of noninvasive ventilation, or high-flow nasal oxygen therapy. We collected the Sequential Organ Failure Assessment score, radiologic score,15 modified CPIS,7 and C-reactive protein level.
The main outcomes were primary pneumonia; recurrent pneumonia15; need for endotracheal mechanical ventilation, noninvasive ventilation, or high-flow nasal oxygen therapy after FOB; occurrence of ARDS; ICU stay; and ICU mortality. Recurrent pneumonia was classified as a relapse if ≥1 of the initial causative bacterial strains grew in a significant concentration from the second FOB samples; if not, recurrent pneumonia was classified as a superinfection.15
Definition of Non-Ventilator ICU-Acquired Pneumonia, Sampling Techniques, and Antimicrobial Treatments
The clinical suspicion of non-ventilator ICU-acquired pneumonia was based on the clinical criteria used in CPIS calculation6 (ie, a new or persistent infiltrate by chest radiography combined with at least one of the following: temperature ≥38.5°C or <36.5°C, leukocyte count >11,000/mm3, hypoxemia with PaO2/FIO2<240, and purulent tracheal secretions). The following microbiological sampling procedures were performed routinely. A physiotherapist obtained spontaneous sputum and evaluated its quality. Spontaneous sputum samples were considered valid if they contained >25 granulocytes and <10 epithelial cells under ×100 magnification. FOB was performed by the attending senior physician. An FOB-EA specimen was collected first via a sputum suction trap (Vygon, Ecouen, France), and the FOB channel was then rinsed. BAL was performed by infusing 5 20-mL aliquots of sterile normal saline into the lung region where the radiograph showed a new or persistent pulmonary infiltrate, as described previously.1 Only Gram-stained smears and quantitative cultures were performed routinely. Two blood samples for cultures were also obtained in each subject. Culture cutoffs for confirming non-ventilator ICU-acquired pneumonia were ≥107 cfu/mL for spontaneous sputum16 and ≥105 cfu/mL for FOB-EA.9 For FOB-BAL (ie, the reference standard), the cutoffs were >4% of recovered cells containing intracellular bacteria by direct examination and/or ≥104 cfu/mL by culture.3
Results of direct microscopic examinations were used to decide to treat and to guide the initial choice of antibiotics when specimens were positive.17 Unless there was both a low clinical suspicion for non-ventilator ICU-acquired pneumonia and negative microscopy of a lower-respiratory-tract sample, empiric antimicrobial therapy was started.1 When the subject presented signs of severe sepsis, antibiotic therapy was also started, whatever the results of direct microscopic examination.1,17 The antimicrobial treatment policy consisted in prescribing the narrowest-spectrum antibiotic possible for 7 d. Empirical therapy was considered appropriate when the bacteria were susceptible to ≥1 of the antibiotics used. For non-fermenting Gram-negative bacteria, an aminoglycoside alone was considered inappropriate.
Statistical Analysis
Data were entered and analyzed using Statview 5.0 software (SAS Institute, Cary, North Carolina). Normality of data distribution was assessed using the Kolmogorov-Smirnov test. Continuous variables were described as mean ± SD or median (interquartile range) and compared using the Student t test or the Mann-Whitney U test, as appropriate. The chi-square test or Fisher exact test was chosen to compare categorical variables. The results of spontaneous sputum or FOB-EA cultures were compared with those of FOB-BAL cultures using the McNemar test. Sensitivity, specificity, and predictive values were computed using standard formulas.18 P < .05 was considered significant. We expected FOB-BAL to have 85% sensitivity,3 and we therefore assumed that the lower boundary of the 95% CI for FOB-EA would be no lower than 65%. According to previously published sample-size tables,19 we needed at least 52 subjects with and 52 without non-ventilator ICU-acquired pneumonia, assuming a 50% prevalence of non-ventilator ICU-acquired pneumonia.
Results
During the 14-month study period, 859 patients were admitted to our cardiothoracic surgery ICU, and of these, 105 (12.2%) met our study selection criteria. Figure 1 reports the procedures performed in each subject. Non-ventilator ICU-acquired pneumonia was diagnosed in 57 (54.3%) subjects, at a median of 3 (interquartile range 2–5) d after ICU admission. Of these 57 diagnoses, 54 were established by FOB-BAL, 2 by FOB-EA only, and 1 by spontaneous sputum only. The incidence of non-ventilator ICU-acquired pneumonia was 57/859 (6.3%, 95% CI 4.7–7.9%). A modified CPIS >6 had 42% sensitivity (95% CI 29–56) and 87% specificity (95% CI 74–95) for non-ventilator ICU-acquired pneumonia.
Procedures performed in subjects with suspected non-ventilator ICU-acquired pneumonia (NV-ICUAP). *: In 1 subject, confirmed by a spontaneous sputum culture ≥107 cfu/mL. †: In 54 subjects, confirmed by a bronchoalveolar lavage (BAL) culture ≥104 cfu/mL. ‡: In 2 subjects, confirmed by an endotracheal aspirate (EA) culture ≥105 cfu/mL. FOB = fiberoptic bronchoscopy.
Table 1 lists the main characteristics of the subjects with and without non-ventilator ICU-acquired pneumonia, and Table 2 lists the diagnostic criteria, signs of severity, and previous antibiotic use at diagnosis. No subjects had bacteremia concomitantly with non-ventilator ICU-acquired pneumonia.
Characteristics of Subjects at ICU Admission
Clinical Diagnostic Criteria, Signs of Severity, and Previous Antibiotic Use at the Time of Diagnosis
Diagnostic Accuracy of FOB-EA and Spontaneous Sputum Culture
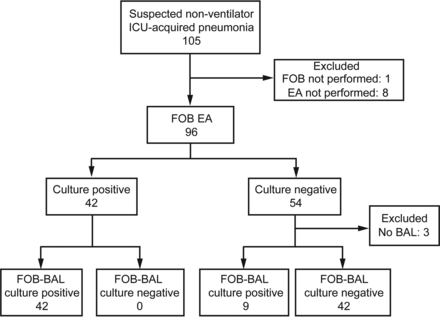
FOB-EA and FOB-BAL smear microscopy.
Both FOB-EA and FOB-BAL specimens were available for 93 subjects (Fig. 2). Smear microscopy of both specimens was negative in 32 subjects and positive in 54 subjects; of the remaining 7 subjects, 5 had a positive FOB-EA and negative FOB-BAL smear (4 had positive FOB-BAL cultures), and 2 had a negative FOB-EA and positive FOB-BAL smear (2 had positive FOB-BAL cultures).
Flow chart of evaluation of endotracheal aspirates (EA) obtained during fiberoptic bronchoscopy (FOB). BAL = bronchoalveolar lavage.
Microbiological cultures of FOB-EA and FOB-BAL specimens.
With FOB-BAL culture as the reference standard, FOB-EA culture had 82% sensitivity (95% CI 69–91%) and 100% specificity (95% CI 89–100%). The positive and negative predictive values of FOB-EA for non-ventilator ICU-acquired pneumonia were 100% (95% CI 89–100%) and 82% (95% CI 69–91%), respectively. FOB-EA culture was significantly less sensitive than FOB-BAL culture (P < .004).
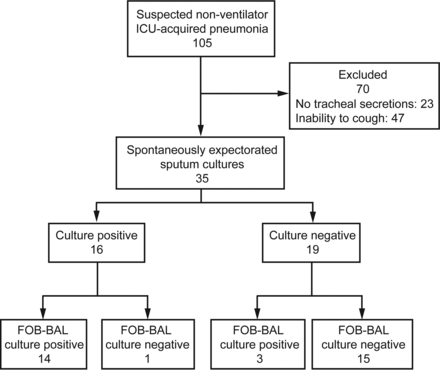
Spontaneous sputum and FOB-BAL smear microscopy.
Spontaneous sputum was obtained from 35 (33%) subjects, and all 35 specimens met our quality criteria. FOB-BAL was available for 33 of the 35 subjects with spontaneous sputum (Fig. 3). Of these 33 subjects, 9 had negative and 16 had positive microscopic smear results for both specimens; of the remaining 8 subjects, 4 had positive spontaneous sputum and negative FOB-BAL smears (1 had a positive FOB-BAL culture), and 4 had negative spontaneous sputum and positive FOB-BAL smears (2 had positive FOB-BAL cultures).
Flow chart of subjects included in the evaluation of spontaneously expectorated sputum specimens. FOB-BAL not performed in 2 subjects. FOB-BAL = bronchoalveolar lavage performed during fiberoptic bronchoscopy.
Microbiological cultures of spontaneous sputum and FOB-BAL specimens.
With FOB-BAL culture as the reference standard, spontaneous sputum culture had 82% sensitivity (95% CI 56–95%) and 94% specificity (95% CI 68–100%). The positive and negative predictive values of spontaneous sputum for non-ventilator ICU-acquired pneumonia were 93% (95% CI 66−100%) and 83% (95% CI 58–95%), respectively. Spontaneous sputum culture showed a trend toward lower sensitivity compared with FOB-BAL culture (P = .004).
Impact of Diagnostic Methods on Microbiological Findings
In 12 subjects (21.0%), non-ventilator ICU-acquired pneumonia was polymicrobial. FOB-EA and FOB-BAL recovered the same organism in 53 subjects: Haemophilus influenzae (n = 14, 26%), Enterobacteriaceae (n = 7, 13%), oropharyngeal flora (n = 7, 13%), Streptococcus pneumoniae (n = 6, 11%), Candida species (n = 5, 9%), Staphylococcus aureus (n = 5, 9%), Pseudomonas aeruginosa (n = 4, 7%), Branhamella catarrhalis (n = 4, 7%), and Staphylococcus epidermidis (n = 1, 2%). Twelve organisms were detected by FOB-BAL but not by FOB-EA: oropharyngeal flora (n = 3); Enterobacteriaceae (n = 3), P. aeruginosa (n = 2), H. influenzae (n = 1), B. catarrhalis (n = 1), Streptococcus mitis (n = 1), and Candida koseri (n = 1). Three organisms were detected by FOB-BAL but not by spontaneous sputum: oropharyngeal flora (n = 1), Escherichia coli (n = 1), and P. aeruginosa (n = 1). Among subjects with Candida species, all but one also had a pathogenic bacterium.
Alternative Diagnoses and Complications of FOB
Of 104 subjects who underwent FOB, 37 (35.6%) developed respiratory complications related to the procedure: 14 (13.5%) had sustained hypoxemia (including 11 receiving noninvasive ventilation or high-flow nasal oxygen therapy and 3 receiving nasal oxygen), 15 (14.4%) required noninvasive ventilation or high-flow nasal oxygen initiation (including 2 without confirmed non-ventilator ICU-acquired pneumonia), and 8 (7.7%) required mechanical ventilation (including 1 without confirmed non-ventilator ICU-acquired pneumonia). In the 48 subjects without non-ventilator ICU-acquired pneumonia, the following 51 diagnoses were established: pulmonary edema (n = 22), atelectasis (n = 16), aspiration pneumonitis (n = 4), pleural effusions (n = 4), pulmonary embolism (n = 2), pulmonary contusion (n = 2), and intra-alveolar hemorrhage (n = 1).
Outcomes of Subjects With and Without Non-Ventilator ICU-Acquired Pneumonia
Empirical antibiotic therapy was started on the day non-ventilator ICU-acquired pneumonia was first suspected in 51 subjects (90%) with non-ventilator ICU-acquired pneumonia and 11 (23%) without non-ventilator ICU-acquired pneumonia (P < .001). Initial empirical antibiotic therapy was adequate in 47 subjects (82%) with non-ventilator ICU-acquired pneumonia. The antibiotic regimen was adjusted based on culture and/or susceptibility-test results in 37 subjects (65%) with non-ventilator ICU-acquired pneumonia and was stopped in all subjects without non-ventilator ICU-acquired pneumonia. Antibiotic treatment duration was 7.6 ± 2.6 d in subjects with non-ventilator ICU-acquired pneumonia compared with 1.6 ± 1.2 days in those without non-ventilator ICU-acquired pneumonia (P < .001). Table 3 reports the main outcomes, which were not significantly different between the 2 groups, except for the use of noninvasive ventilation or high-flow nasal oxygen after FOB.
Outcomes
Discussion
Our study suggests that FOB-EA performs less well than does FOB-BAL for diagnosing non-ventilator ICU-acquired pneumonia. In patients at risk for poor tolerance of FOB, spontaneous sputum may be a reasonable alternative. Importantly, subjects whose culture results were negative for non-ventilator ICU-acquired pneumonia experienced favorable outcomes without prolonged antibiotic treatment.
The incidence of non-ventilator ICU-acquired pneumonia depends on the diagnostic criteria used and patient population. Thus, non-ventilator ICU-acquired pneumonia has been reported in 10.7% of subjects in a medical-surgical cardiothoracic ICU20 and in 14–25% of subjects after lung surgery.21
We found that the modified CPIS had low diagnostic accuracy for non-ventilator ICU-acquired pneumonia, in keeping with data on VAP.7 Therefore, quantitative cultures of pulmonary secretions may improve the identification of patients with true non-ventilator ICU-acquired pneumonia. Diagnostic strategies have been extensively studied in VAP and community-acquired pneumonia but not in non-ventilator ICU-acquired pneumonia.2,4,5,21 The best method for collecting lower-respiratory-tract secretions remains unclear. The European Respiratory Society stated that “bronchoscopic sampling of the lower respiratory tract can be considered in selected non-intubated patients, where gas exchange status allows.”8 As expected, the methods used to diagnose non-ventilator ICU-acquired pneumonia vary widely. One group routinely performed FOB to obtain pulmonary samples,21 whereas only 44% of EOLE study subjects underwent FOB.4 The diagnostic usefulness of protected specimen brush sampling has been established in both intubated and non-intubated subjects.3,9,14,17 This technique is both sensitive and specific for identifying microorganisms.3 However, contrary to BAL, Gram staining of protected specimen brush samples only partially identified microorganisms growing at significant concentrations.22 Finally, BAL is known to collect material from a far larger lung region and therefore has greater sensitivity compared with protected specimen brush sampling.3 In a German study,5 BAL was performed in 13.2% and endotracheal aspirate in 40% of subjects. FOB-EA had lower diagnostic accuracy than did FOB-BAL in our study. Similarly, in studies of VAP, FOB-BAL performed better than FOB-EA, although some results were conflicting.3 However, when FOB-BAL is not feasible, FOB-EA is a good alternative. Spontaneous sputum also deserves consideration based on our results. In keeping with earlier data,13 only 33% of our subjects were able to produce spontaneous sputum, a fact that considerably limits the usefulness of this sampling technique. Moreover, the culture yield was substantially higher with FOB-EA or FOB-BAL compared with spontaneous sputum.13,14 Definitive conclusions regarding the role for spontaneous sputum in diagnosing non-ventilator ICU-acquired pneumonia will require larger studies providing greater statistical power.
In some subjects, BAL cultures recovered microorganisms that might have been contaminants. In particular, Candida pneumonia is rare, and the recovery of Candida species from respiratory-tract secretions is usually not clinically important23 but warrants further investigation.3 In other studies, S. epidermidis was isolated from 1.4–7.0% of cultures3,4,17,24 and considered either nonpathogenic24 or pathogenic.25 The appropriateness of treatment in patients with S. epidermidis should be discussed on a case-by-case basis.
Several microorganisms isolated by FOB-BAL cultures, such as Enterobacteriaceae and P. aeruginosa, were not recovered by FOB-EA or spontaneous sputum, as reported previously in subjects with VAP and community-acquired pneumonia.9,10,13,26 However, endotracheal aspirates have consistently been reported to grow more organisms than do invasive quantitative cultures.10 The lower frequency of positive FOB-EA than FOB-BAL samples in our study may be ascribable to a number of factors, such as inadequate endotracheal aspirate sampling technique, failure of endotracheal aspirates to reflect the organisms present in the lower respiratory tract, and antibiotic treatments. Moreover, variations in endotracheal aspirate culture results have been reported for about 20% of specimens.27 Finally, contamination with oropharyngeal flora is a major limitation to the value of endotracheal aspirate sampling.27 Separating contaminants from pathogenic organisms can be achieved by quantitative BAL cultures but may be more difficult with FOB-EA or spontaneous sputum cultures.16
Oropharyngeal pathogens were recovered in some of the cultures in our study. Penetration of pharyngeal contents into the lower respiratory tract may be facilitated immediately after extubation.28,29 The inhalation of oropharyngeal flora can cause nosocomial pneumonia,25,29 and without treatment, lung cavitation and abscess formation may occur.25,29
Critically ill patients are at high risk for complications during most invasive procedures.30 It is therefore important to demonstrate that the potential benefits of bronchoscopy outweigh the risks. Because many patients are unable to produce spontaneous sputum samples, FOB may be a reasonable option. The rate of FOB complications in our study was consistent with earlier reports. FOB has been associated with respiratory status deterioration in 25% of subjects,12 and mechanical ventilation was required after FOB in about 15% of subjects.12 However, we agree with Cracco et al12 that respiratory status deterioration is more likely to represent the natural progression of the underlying disease than a complication of FOB. Among our subjects without confirmed non-ventilator ICU-acquired pneumonia, only 1 (2%) required mechanical ventilation. Importantly, our results may not apply to patients in hospital wards. In critically ill patients, such as those in our study, FOB should always be performed in a setting that allows close monitoring and emergent intubation and mechanical ventilation if needed.30
One of the main findings from our study pertains to the outcome of subjects without non-ventilator ICU-acquired pneumonia who did not receive antibiotics or whose antibiotics were stopped promptly. The optimal use of antibiotic therapy has been addressed in several studies of subjects with VAP but has not been evaluated in those with non-ventilator ICU-acquired pneumonia. In the EOLE study,4 94% of subjects received antibiotics within 48 h after pneumonia was first suspected. Importantly, negative microbiological results did not lead the physicians to discontinue empirical antibiotic therapy.4 Nevertheless, several studies have confirmed that empirical antibiotics can be safely discontinued after 72 h or completely withheld6,31–33 with no adverse effect on VAP recurrence or mortality in subjects with negative cultures. Invasive diagnostic testing may increase physician confidence and provide valuable guidance for deciding whether to limit or discontinue antibiotic treatment.1,24 The main diagnoses other than non-ventilator ICU-acquired pneumonia in our study were pulmonary edema and atelectasis, as reported previously.34
Nosocomial pneumonia has been associated with increased mortality.1,2,4,21 In previous studies, hospital mortality rates were similar or lower in subjects with non-ventilator ICU-acquired pneumonia compared with those with VAP.2,5,35 The mortality rate was low in our study and did not differ between subjects with and without confirmed non-ventilator ICU-acquired pneumonia. This finding suggests low illness severity and predominant infection by low-risk pathogens.
Our study has several limitations. First, it was conducted in a single center and in a specific postoperative population. Studies in other types of postoperative patients are needed. Second, FOB was available around the clock, which may not be the case in other ICUs. Third, as mentioned previously, the sample size was not computed with the goal of drawing definitive conclusions about spontaneous sputum. Finally, because airway sampling was performed 2 d after extubation, some subjects might have had delayed diagnosis of VAP rather than postextubation pneumonia. However, our team has extensive experience with diagnosing VAP in cardiothoracic surgery patients, and it is very unlikely that VAP diagnoses were missed.
Conclusions
Our results suggest that FOB-EA may perform less well than FOB-BAL for diagnosing non-ventilator ICU-acquired pneumonia. Therefore, BAL should be performed to diagnose non-ventilator ICU-acquired pneumonia when FOB is feasible. Otherwise, spontaneous sputum is a valuable diagnostic tool but can be obtained from only a minority of patients. When bacteriological cultures are negative, antibiotic therapy can be safely stopped. The mortality rate was low in our study and did not differ between subjects with and without confirmed non-ventilator ICU-acquired pneumonia.
Footnotes
- Correspondence: François Stéphan MD PhD, Réanimation adulte, Centre Chirurgical Marie Lannelongue, 133 Avenue de la Résistance, 92350 Le Plessis Robinson, France. E-mail: f.stephan{at}ccml.fr.
This work was supported solely by institutional and departmental sources. The authors have disclosed no conflicts of interest.
Dr F Stéphan presented in part at the American Thoracic Society International Conference 2014, held May 16–21, 2014, in San Diego, California (Abstract A6236).
- Copyright © 2016 by Daedalus Enterprises