Abstract
BACKGROUND: Preterm infants often require some form of respiratory support with supplemental oxygen and are monitored by continuous pulse oximetry (SpO2). The study objective was to determine whether the histogram distribution of SpO2 over a 24-h period will predict readiness for weaning respiratory support in preterm infants. We hypothesize that infants with ≥15% of time spent with SpO2 <86% before transitioning from CPAP or high-flow nasal cannula (HFNC) to low-flow nasal cannula, oxyhood, or room air are more likely to fail transitioning.
METHODS: The SpO2 histograms were downloaded daily for 31 infants, 24–32 weeks gestational age, before transition from CPAP or HFNC to low-flow nasal cannula, oxyhood, or room air. The SpO2 histogram downloads were continued for each infant for 1 week after transition or when the infant reached 36 weeks postmenstrual age or when SpO2 monitoring was discontinued. Failure was defined as an increase in respiratory support within 72 h of transitioning. We compared the percentage of time for each SpO2 quintile for the 24-h periods before and immediately following CPAP/HFNC transitioning between groups.
RESULTS: Twenty-four subjects transitioned successfully, but 7 subjects failed. Two of 7 subjects (28.6%) who failed transition experienced SpO2 <86% ≥15% of the time pretransition compared with none in the success group (P = .045). The failure group experienced SpO2 <86% 10.7 ± 11.9% of time pre-wean compared with 3.3 ± 4.7% of time in the success group (P = .02). In contrast, infants who were successfully weaned tended to experience a greater percentage of time with SpO2 >95% compared with the failure group, both pre-wean (54.3 ± 36.1% vs 33 ± 27.7%, P = .16) and post-wean (52 ± 35.4% vs 27.4 ± 27.7%, P = .10).
CONCLUSIONS: These data suggest that pulse oximetry histograms may be useful in assessing CPAP/HFNC support transition readiness.
Introduction
Preterm infants often require some form of respiratory support with supplemental oxygen. Regulating oxygen exposure is essential because hyperoxia is associated with retinopathy of prematurity and bronchopulmonary dysplasia,1,2 whereas intermittent hypoxia is associated with increased mortality, severe retinopathy of prematurity, and pulmonary hypertension.2–7 Continuous pulse oximetry (SpO2) measured by new generation high-resolution pulse oximeters is more accurate than hand-transcribed SpO2 values, and more episodes of intermittent hypoxia are detected.8,9 Hence, high-resolution pulse oximetry with target O2 ranges has been implemented in many neonatal ICUs to avoid fluctuations in SpO2 and to reduce the risk of adverse outcomes.10–12 Despite these advances, maintaining SpO2 within the targeted range is difficult.13–17
To reduce lung injury from mechanical ventilation, noninvasive modes of respiratory support, such as nasal CPAP and high-flow nasal cannula (HFNC), have been increasingly used.18–23 However, there is no agreement on best practices for weaning these devices.24–27 Noninvasive indicators demonstrating readiness for reducing respiratory support are lacking. As a first step to develop a tool to assess readiness for transition from noninvasive respiratory support, we conducted an observational study to determine whether the pulse oximetry histogram distribution is an objective noninvasive tool to predict readiness for change in respiratory support in preterm infants and avoid SpO2 fluctuations that can be exacerbated during the transition period. We hypothesized that infants with ≥15% of cumulative time spent with SpO2 <86% in the 24-h period before transitioning from CPAP or HFNC to low-flow nasal cannula, oxyhood, or room air are more likely to fail transition.
QUICK LOOK
Current knowledge
To reduce lung injury from mechanical ventilation in preterm infants, noninvasive modes of respiratory support, such as nasal CPAP and high-flow nasal cannula, have been increasingly used. Noninvasive indicators demonstrating readiness for weaning from noninvasive respiratory support are lacking.
What this paper contributes to our knowledge
In a pilot study of preterm infants ≤32 weeks gestation receiving CPAP or high-flow nasal cannula, the percentage of time with SpO2 <86% in the 24 h preceding transition was greater in infants who failed transition compared with those who were successfully weaned off noninvasive respiratory support. Infants who were successfully transitioned tended to experience a greater percentage of time with SpO2 >95% compared with the failure group, both pre- and post-wean.
Methods
This was a prospective, observational cohort study conducted at the University of Maryland Medical Center neonatal ICU from April 2012 to July 2013. The University of Maryland institutional review board approved the study protocol, and parental consent was obtained for each subject. Inclusion criteria were (1) gestational ages 24–32 weeks and (2) receipt of CPAP or HFNC (≥2 L/min) for at least 24 h. Exclusion criteria were (1) positive pressure respiratory support other than CPAP/HFNC; (2) congenital heart disease or pulmonary anomalies; and (3) persistent pulmonary hypertension. Infants who had been receiving mechanical ventilation support were screened for eligibility once they were extubated to CPAP or HFNC.
Respiratory management of all study infants was provided according to the University of Maryland Medical Center neonatal ICU guidelines for criteria for mechanical ventilation, extubation, CPAP and HFNC initiation, and low-flow nasal cannula and oxyhood use. Mechanical ventilation is instituted for respiratory failure (PaO2 <50 with FIO2 ≥0.60 and/or PaCO2 >55), and extubation to CPAP or HFNC is considered once positive inspiratory pressure is ≤18 cm H2O, PEEP is 4–5 cm H2O, the intermittent mandatory ventilation rate is ≤15, and the FIO2 is <0.3. CPAP or HFNC is used as the primary mode of noninvasive respiratory support if PaCO2 is <55 mm Hg, FIO2 is <0.6, and <6 apnea/bradycardia/desaturation episodes occur within 6 h requiring stimulation. Transition from CPAP or HFNC is considered once the infant reaches CPAP of 4–5 cm H2O or HFNC of 2 L/min, FIO2 <0.3 and not increasing, no significant chest retractions, and infrequent apnea/bradycardia/desaturation episodes. It is our neonatal ICU practice to transition off CPAP or HFNC with the intention to remain off support. CPAP support was provided by the VIASYS Infant Flow SiPAP System (CareFusion, San Diego, California) and HFNC by Precision Flow (Vapotherm, Exeter, New Hampshire).
For the study, the attached Nellcor OxiMax N-600x pulse oximeter (Covidien, Mansfield, Massachusetts) was set to both neonatal and normal modes, where data were gathered every 4 s with an averaging time of 5–7 s. The target oxygen saturation range used was set by the current University of Maryland Medical Center neonatal ICU postmenstrual age-based targeted oxygen saturation protocol, where targeted SpO2 is 88–93% for infants <33 weeks postmenstrual age and 90–97% for infants ≥33 weeks postmenstrual age. The histogram distribution of pulse oximetry displaying the percentage of time spent in the pulse oximeter preset oxygen saturation ranges (96–100, 91–95, 86–90, 81–85, and <80%) for a 24-h period was recorded daily until the infants were transitioned to low-flow nasal cannula (≤1.5 L/min), oxyhood, or room air for 1 week post-transition or until the infant reached 36 weeks postmenstrual age without attempted transition. The primary outcome was transition success defined as transition to low-flow nasal cannula, room air, or oxyhood for 72 h. If a subject was restarted on CPAP or HFNC by the clinical team within 72 h after transitioning to low-flow nasal cannula/oxyhood/room air, the subject was considered a transition failure. The clinical team was blinded to the 24-h histogram distributions, and these data were not incorporated into clinical decision-making. The clinical team made all respiratory care decisions concerning timing of CPAP/HFNC transition to lower respiratory support as well as subsequent decisions to increase respiratory support post-transition based on neonatal ICU guidelines.
Data collection included demographic and clinical variables, such as presence of patent ductus arteriosus; antenatal steroids; surfactant administration; average percentage of FIO2 over 24-h intervals; documented apnea, bradycardia, and/or desaturation events; and clinical outcomes, such as retinopathy of prematurity, necrotizing enterocolitis, intraventricular hemorrhage, and bronchopulmonary dysplasia defined as receipt of supplemental oxygen or positive pressure support at 36 weeks postmenstrual age. For infants receiving low-flow nasal cannula, the effective FIO2 was calculated using the STOP-ROP tables.28
The sample size of 30 subjects for the primary outcome was chosen in order to provide adequate numbers to determine whether the SpO2 histogram pattern for babies who fail transition from CPAP/HFNC to low-flow nasal cannula/oxyhood/room air differs from the SpO2 histogram pattern of infants who make a successful transition. We selected <86% SpO2 as a clinically important level of hypoxemia. Since studies of high-resolution oximetry indicate that SpO2 in the hypoxemic range occurs <15% of the time during noninvasive respiratory support with active O2 target range management,8,29 we selected a cut-off of ≥15% of time with SpO2 <86% as high-risk for transition failure. In a preliminary sample of 5 infants <33 weeks postmenstrual age at our neonatal ICU, 2 infants (40%) experienced SpO2 <86% >15% of the time during the transition from CPAP to low-flow nasal cannula (unpublished data). For the sample size, we assumed that one third of the subjects would have ≥15% of time spent with SpO2 <86% when transitioning off CPAP/HFNC and that 75% of those babies would fail transitioning. With these assumptions, a sample size of 30 was determined to be sufficient with a power = 0.848 and an α = 0.05 to detect a difference if the failure rate for infants who have ≤15% of time with SpO2 <86% is 15%.
The mean ± SD of the percentage of time for each SpO2 quintile over time for each subject was calculated for each period of respiratory support. The percentage of time for each SpO2 quintile for the 24-h period pre- and post-transition from CPAP/HFNC to low-flow nasal cannula/oxyhood/room air was compared between the successfully transitioned and transition failure groups. Demographic and respiratory variables were compared between the groups by chi-square test for categorical variables and t test for continuous variables. Receiver operating characteristic curve analysis was done to assess the sensitivity and specificity of different percentage of time cut-offs for SpO2 <86%. The Spearman's rank correlation test was used to calculate the correlation coefficient between percent change in FIO2 and the percentage of time with SpO2 <86% and SpO2 >95% for the 24-h periods pre- and post-transition.
Results
Of 137 infants admitted to the University of Maryland Medical Center neonatal ICU with gestational ages of 24–32 weeks, 53 were eligible for the study, and consent was obtained from the 35 eligible subjects who were approached for consent (Fig. 1). Eighteen eligible subjects were missed due to unavailability of the investigators. Four subjects were not included in the final analysis due to missing data (n = 2), transfer to another facility before transition (n = 1), and no transition attempt before 36 weeks postmenstrual age (n = 1). Seven of the 31 subjects were ≥33 weeks postmenstrual age at the time of transition. Twenty-four subjects transitioned successfully from CPAP/HFNC, but 7 subjects failed transition. In all cases of failed transition, CPAP was reinstituted due to increased work of breathing assessed as an increased severity of subcostal and intercostal retractions, nasal flaring, and poor air entry on auscultation. All 7 failed subjects transitioned from CPAP; 6 infants were transitioned from CPAP +4 cm H2O, and 1 infant was transitioned from CPAP +5 cm H2O. There were a total of 8 subjects transitioned from HFNC, all in the success group, 7 infants transitioned from 2 L/min and 1 from 3 L/min. There were 16 babies who transitioned successfully from CPAP, 14 infants transitioned from CPAP +4 cm H2O, and 2 babies from CPAP +5 cm H2O. The failed transition subjects were less mature but had similar birth weights compared with the successful group (Table 1). The groups were similar for Apgar scores, exposure to antenatal steroids, surfactant, and patent ductus arteriosus treated with either indomethacin or surgical ligation. Both groups experienced similar rates of retinopathy of prematurity of at least Stage 2, intraventricular hemorrhage, and bronchopulmonary dysplasia at 36 weeks postmenstrual age.
Flow chart. Weaning success was defined as weaning to low-flow nasal cannula, room air, or oxygen hood for 7 d. Weaning failure was defined as returning to CPAP or high-flow nasal cannula within 72 h.
Characteristics of Study Subjects
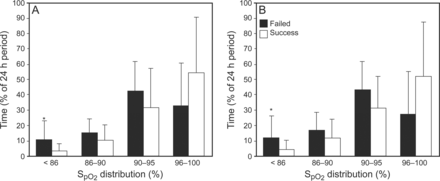
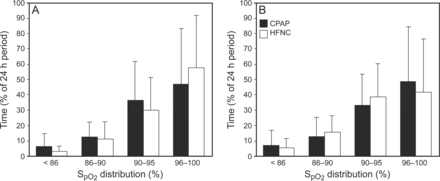
At the time of CPAP/HFNC transition, there was no significant difference in postmenstrual age or body weight between the groups (Table 2). Two of the 7 subjects (29%) in the failure group experienced SpO2 <86% ≥15% of the time pre-wean compared with none in the successful group (P = .045). The failure group experienced SpO2 <86% 11 ± 12% of the time pre-wean compared with 3 ± 5% of time in the successful group (P = .02) (Fig. 2). A receiver operating characteristic curve was generated, revealing that SpO2 <86% ≥15% of time had 29% sensitivity and 96% specificity for wean failure, whereas a cut-off of ≥10% of time had 43% sensitivity and 83% specificity. Infants who were successfully transitioned tended to experience a greater percentage of time with SpO2 >95% compared with the failure group, both pre-wean (54 ± 36 vs 33 ± 28%, P = .16) and post-wean (52 ± 35 vs 27 ± 28%, P = .10) (Fig. 2). There was no difference in effective FIO2 or breathing frequency between the successful and wean failure groups both pre- and post-wean (Table 2). In addition, there was no statistical difference in SpO2 histogram distribution between CPAP or HFNC subjects pre- or post-wean (Fig. 3) and between infants <33 weeks and ≥33 weeks postmenstrual age pre- or post-wean (data not shown). However, as the percentage of time SpO2 <86% pre-transition increased, the percentage change in effective FIO2 post-transition increased (ρ = 0.585, P = .01) (Fig. 4A). Conversely, as the percentage of time SpO2 >95% pre-transition increased, the percentage change in the effective FIO2 decreased (ρ = −0.431, P = .035) (Fig. 4B).
Comparison of Respiratory Variables in Success and Failure Groups Pre- and Post-Transition
Comparison of SpO2 histogram distribution pre-transition (A) and post-transition (B) between successful and failed transition groups. SpO2 histogram was recorded for the 24-h periods pre- and post-transition from either CPAP or high-frequency nasal cannula to low-frequency nasal cannula or room air. Data are expressed as mean ± SD. * P < .05.
Comparison of SpO2 histogram distribution pre-transition (A) and post-transition (B) of subjects transitioned from CPAP or high-frequency nasal cannula (HFNC) to low-frequency nasal cannula or room air. Data are expressed as mean ± SD.
Correlation between percent change in FIO2 post-transition and percentage of time with SpO2 <86% (A) and percentage of time with SpO2 >95% (B). For each subject, the percentage change in FIO2 post-transition was calculated. The Spearman's rank correlation test was used to calculate the correlation coefficient between percent change in FIO2 and percentage of time with SpO2 <86% and SpO2 >95% for the 24-h periods pre- and post-transition.
Discussion
Noninvasive respiratory support is commonly used in the management of respiratory distress even in the smallest infants. Recent randomized control trials have indicated that early CPAP may be an effective alternative to prophylactic surfactant administration.18,20,21,23 When compared with prophylactic or early surfactant therapy studies, early CPAP results in lower rates of bronchopulmonary dysplasia/death.18,21,23 HFNC has been increasingly used to provide primary respiratory therapy initiated at birth, as postextubation support, or transitioning from CPAP. However, large randomized trials have not been performed comparing efficacy of HFNC with that of nasal CPAP.19
Because methods of weaning CPAP vary in clinical practice, several studies have evaluated alternative CPAP weaning protocols.24–27 In a multi-center, randomized control trial comparing 3 CPAP weaning protocols, Todd et al24 demonstrated that decreasing CPAP pressure rather than cycling time off CPAP is the most effective method for CPAP weaning.24 The group “taken off CPAP with the view to stay off” had significantly shortened CPAP weaning time, oxygen duration, incidence of bronchopulmonary dysplasia, and duration of hospitalization compared with cycling on/off CPAP with incremental time off CPAP with or without low-flow nasal cannula support when off CPAP. In this current study, all subjects were transitioned off CPAP of +4–5 cm H2O or HFNC of 2 L/min with the intention to remain off support.
In the trial by Todd et al,24 infants were randomized to a weaning method once stability criteria were reached. The stability criteria included CPAP of 4–6 cm H2O, FIO2 <0.25 and not increasing; no significant chest retractions; <3 episodes of self reverting apnea and/or bradycardic and/or desaturation episodes/h for the previous 6 h; not currently treated for PDA or sepsis; tolerating time off CPAP during care; and an average SpO2 >86% most of the time or PaO2/transcutaneous PaO2 >45 mm Hg.24 Subjects in our study met these stability criteria, but we assessed whether adding the specific criterion of percentage of time with SpO2 <86% in the 24-h period before anticipated respiratory support wean better predicted transition failure. The high specificity of the SpO2 <86% >15% of time cut-off by the receiver operating characteristic analysis suggests that infants who experience SpO2 <86% for <15% of time pre-transition are likely to successfully transition from noninvasive positive pressure support. In addition, we observed that infants who were successfully weaned spent a greater percentage of time with SpO2 >95% than the wean failure group, suggesting that these infants may have been successfully weaned earlier and avoided hyperoxia exposure. The SpO2 histogram also predicted post-transitional changes in oxygen requirements. With increasing percentage of time with SpO2 <86% pre-transition, there was an increase in FIO2 post-transition, and increasing percentage of time with SpO2 >95% was associated with reduced FIO2 post-transition.
There are a number of limitations of our study. Due to the observational nature of the study, decisions concerning timing to transition off CPAP/HFNC and reinstituting respiratory support were made by the clinical team using neonatal ICU guidelines rather than by prespecified study criteria. The clinical team preference determined whether CPAP or HFNC was used for noninvasive respiratory support. Although these modes may improve gas exchange by different mechanisms, similar compliance and work of breathing have been noted in prior crossover studies of a range of CPAP/HFNC support.30,31 In this study, there were no differences in SpO2 histogram distributions between CPAP and HFNC groups pre- and post-wean. In addition, there was overlap of the highest histogram quintile (>95%) of the Nellcor oximeter and the target O2 saturation range for the ≥33 weeks postmenstrual age (90–97%). This is an unavoidable limitation of our study, since the oximeter ranges are fixed, and the target ranges are set by clinical practice. However, predicting wean failure as percentage of time spent with SpO2 <86% avoids this limitation, since there is agreement with the lower 2 quintiles (<80% and 81–85%) for the target oxygen saturations for infants <33 weeks and ≥33 weeks postmenstrual age. Although there was a greater difference between the selected SpO2 <86% cut-off and the lower SpO2 target for infants ≥33 weeks (90%) and <33 weeks postmenstrual age (88%), we could not determine the impact of this difference on the predictive value of time <86% SpO2, since the histogram distributions were similar for the infants in the different SpO2 target ranges. The clinical teams could view the real-time SpO2 but not the downloaded 24-h histograms, and this information was not incorporated into clinical care decisions.
Conclusions
In summary, the current study suggests that the pulse oximetry histogram combined with clinical stability criteria may better determine readiness for transitioning off CPAP/HFNC with an ultimate goal of preventing transition failures. Future trials of stability criteria incorporating SpO2 histogram data are warranted to better identify readiness for noninvasive respiratory support transition.
Footnotes
- Correspondence: Hyung Chul Woo MD, University of Maryland School of Medicine, 110 South Paca Street, 8th Floor, Baltimore, MD 21201. E-mail: hwoo{at}peds.umaryland.edu.
Dr Mascoll-Robertson presented a version of this paper at the Pediatric Academic Societies/Society for Pediatric Research Annual Meeting, held May 4–7, 2013, in Washington, DC.
The authors have disclosed no conflicts of interest.
See the Related Editorial on Page 569
- Copyright © 2016 by Daedalus Enterprises