Abstract
Airway pressure release ventilation (APRV) was originally described as a mode to treat lung-injured patients with the goal to maintain a level of airway pressure that would not depress the cardiac function, deliver mechanical breaths without excessive airway pressure, and to allow unrestricted spontaneous ventilation. Indeed, based on its design, APRV has technological features that serve the goals of safety and comfort. Animal studies suggest that APRV leads to alveolar stability and recruitment which result in less lung injury. These features are sought in patients at risk for lung injury or with ARDS. APRV allows unrestricted spontaneous ventilation, which is welcome in the era of less sedation and increased patient mobility (the effects in terms of lung injury remain to be explored). However, we must highlight that the performance of APRV is dependent on the operator-selected settings and the ventilator's performance. The clinician must select the appropriate settings in order to make effective the imputed benefits. This is a challenge when the ventilator's performance is not uniform, and the outcomes depend on high precision settings (very short expiratory time), where small variations can lead to undesired outcomes (de-recruitment or large tidal volumes leading to lung injury). Finally, we do not have evidence that APRV (as originally described) improves relevant clinical outcomes of patients with ARDS. For APRV to become the primary mode of ventilation for ARDS, it will require development of sound protocols and technological enhancements to ensure its performance and safety. For now, APRV does have a greater potential for adversely affecting patient outcome than improving it; unless definitive data are forthcoming demonstrating outcome benefits from the use of APRV in ARDS, there is no reason to consider this approach to ventilatory support.
- Airway pressure release ventilation
- biphasic positive-pressure breathing
- lung injury
- work of breathing
- auto PEEP
Introduction
Should we use airway pressure release ventilation (APRV) as the primary mode of mechanical ventilation in ARDS? To answer this question we will define the clinical goals, the technical features of the mode, and the evidence available. The main goal of this article is to critically evaluate the pros and cons of using APRV as the main mode of ventilation for ARDS.
What Is APRV?
APRV is a mode of mechanical ventilation that was described for the first time by Stock and Downs in 1986.1 In their initial description, the pressure was alternated from a high level to a low level with a switch while the patient breathed spontaneously between the pressure changes. They termed it as 2 levels of CPAP (Phigh and Plow) because they built a prototype using CPAP valves. Current ventilators use closed-loop control of an active expiratory valve to allow spontaneous breaths and keep the airway pressure constant. In terms of nomenclature,2 APRV is defined as a pressure control (PC) mode where there are 2 types of breaths: (1) mandatory breaths (time-triggered and time-cycled), when there is a mandatory step change in pressure (inspiratory time is called Thigh, and expiratory time is called Tlow) and (2) spontaneous breaths (patient-triggered and patient-cycled), where the timing and breath size are determined by the patient. In the classic description of APRV, the targeting scheme was set-point3 and the ratio of inspiratory time to expiratory time (I:E) was inverse (4:1). Thus, classic APRV can be described as inverse ratio PC-intermittent mandatory ventilation (IMV), with unrestricted spontaneous breathing. Originally, APRV did not provide any attempt to synchronize the mandatory to the spontaneous breaths or provide any assistance (in the form of pressure support or tube compensation) to the spontaneous breaths.1,4
In 1989, Baum et al,5 described a similar concept, termed biphasic positive pressure ventilation, essentially describing any form of pressure control where spontaneous breathing was allowed during the mandatory breath, although the I:E was not inverted (Fig. 1). The literature on APRV/biphasic increased rapidly after its initial description. By the mid-1990s, the mode was available in some critical care ventilators, and now almost all ventilators (although using different mode names) allow the delivery of inverse ratio- PC-IMV.6 Thus, the mode is commonly available on intensive care ventilators, and multiple claims and uses have been reported.
Graphic representation of pressure control-continuous mandatory ventilation (CMV), pressure control-intermittent mandatory ventilation (IMV), pressure control-intermittent mandatory ventilation with inverse ratio (airway pressure release ventilation; APRV), biphasic ventilation, and CPAP. One can notice the differences in spontaneous breaths during inspiration and expiration and the inspiratory-expiratory ratios. The most frequent source of confusion in the literature is to combine APRV and biphasic ventilation in the same group. From Reference 4, with permission.
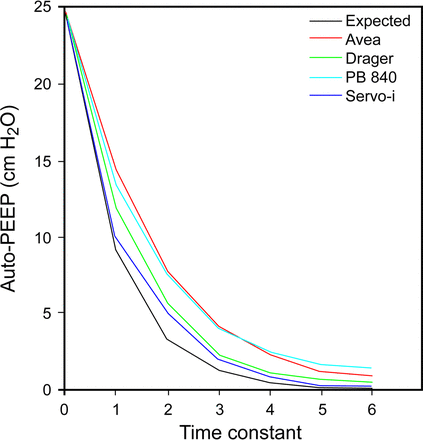
Unfortunately, there are 4 problems that make the evaluation of the clinical evidence difficult. (1) The terms biphasic and APRV are used indistinctly7; studies may state that APRV or biphasic was applied when the I-E ratio was not consistent with the definition. This has evident implications in the outcomes of these studies. Figure 1 highlights the different variations of pressure control ventilation. (2) Not all ventilators deliver the same form of inverse ratio PC-IMV with unrestricted spontaneous breathing. Some ventilator manufacturers implemented algorithms to synchronize spontaneous breaths to the start or end of the mandatory breath (Phigh) or allowed the application of pressure support of the spontaneous breaths (during Phigh and Plow). The effects of these modifications remain to be described. (3) Not all mechanical ventilators perform equally, particularly when trying to set the release time, Tlow,8,9 because the amount of air trapping varies significantly between ventilators (Fig. 2). Finally, (4) there is no standardized protocol for setting the ventilator used by all research groups. For example, some groups use very short Tlow to generate auto-PEEP, whereas others allow full exhalation. In summary, although there are clinical data on the use of APRV, the data are difficult to analyze and compare, given the above reasons. To make our analysis, we will focus on the technical characteristics and evidence of APRV as classically described (ie, using inverse I-E ratio).
Comparison of the expected auto-PEEP (calculated) with the performance of 4 ventilators and a time constant of 0.3 s. Among 4 different ventilators with the same settings, the flow decay curve and the resulting auto-PEEP are substantially different. From Reference 8, with permission.
Pro Position
APRV is inverse ratio pressure control where the patient is allowed to breathe spontaneously at any point in the ventilatory cycle. Because it is a pressure control mode, the ventilator will aim to maintain the pressure constant at the set levels (ie, Phigh and Plow). In pressure control modes, the effect of changes in lung compliance or resistance and the presence of respiratory effort will result in changes in flow and tidal volume (VT) while the ventilator-delivered pressure remains constant. In APRV, the patient-ventilator interaction is determined by a set-point targeting scheme,3 which is the most basic form of closed-loop control. In set-point targeting, the ventilator will do what the operator sets with no targets set automatically by the ventilator. It follows that the performance of APRV is completely dependent on the operator's skill in selecting optimum settings.
When a clinician places a patient on mechanical ventilation, he or she has certain goals to achieve. These goals, although many, fall into 3 groups10: safety (ensuring gas exchange and preventing lung injury), comfort (optimizing patient-ventilator synchrony and work of breathing [WOB]), and liberation (decreasing the duration of mechanical ventilation). Although these goals are concurrent (we always want a patient to be safe, comfortable, and closer to liberation), one of these goals takes precedence over the others at any point in time. We will use these goals as the framework to analyze the technical features of APRV. The reader is reminded that this section assumes perfect ventilator performance and a ventilator operator who selects optimal settings.
APRV and Safety
The technical features, in terms of safety, where APRV may benefit patients with lung injury are described below.
Higher Mean Airway Pressures for a Given Minute Ventilation and Peak Airway Pressure.
APRV, due to the inverse I-E ratio, will have higher mean airway pressure (P̄aw) than conventional lung-protective ventilation (both pressure or volume control) with a normal I-E ratio of 1:2–3.
P̄aw is calculated11 as: P̄aw = [(TI × Ppeak) + (TE × PEEP)]/(TI + TE) or, using the terminology common to APRV: P̄aw = [(Thigh × Phigh) + (Tlow × Plow)]/(Thigh + Tlow). In APRV, Thigh is much longer than Tlow, and thus P̄aw will be higher for the same peak airway pressure when compared with conventional ventilation.12 In APRV, the time spent at the higher pressure is generally 80–90% of the respiratory cycle. The higher the P̄aw, within a reasonable range, the higher PaO2 will be, due to alveolar recruitment.13–15 Similarly, with a longer Thigh, the alveolar mixing time lengthens and dead space decreases, potentially leading to a decrease in PaCO216,17 For these reasons, in the setting of ARDS (where surfactant deactivation, atelectasis, and edema of the alveoli are present), the higher P̄aw should be beneficial. The clinical importance is that for a given oxygenation target, APRV will provide a lower peak airway pressure compared with conventional ventilation,18 which may translate into a lower peak transpulmonary pressure (this is mainly under passive conditions). This feature of APRV serves the goal of safety because it optimizes gas exchange and potentially reduces the risk of lung injury.
Ventilation Occurs With Potentially Less Risk of Ventilator-Induced Lung Injury.
In both conventional pressure control ventilation and APRV, the VT is generated by the difference in pressure from the PEEP, or Plow, to the set inspiratory pressure or, Phigh (Fig. 3). Figure 3 demonstrates conventional mechanical ventilation and APRV superimposed on a pressure-volume curve. We can observe that in order to achieve the same mean lung volume, conventional ventilation (with its lower I:E) would require a higher PEEP; thus, the delivery of the same VT will have a higher end-inspiratory volume and hence an increased risk of injury by overdistention. Smith et al19 used a computational model linked to airway pressure and flow to demonstrate both tissue overdistention (indicative of risk for volutrauma) and repetitive recruitment (indicative of risk for atelectrauma) in a passive mouse model of ARDS. They found that if the Tlow duration is such that the terminal (end-expiratory) flow is ≥75% of peak expiratory flow (PEF), the result is a higher auto-PEEP and alveolar recruitment without any additional overdistention. Importantly, if the Tlow is not set appropriately, there will be lower auto-PEEP and higher cyclic recruitment than in conventional ventilation.
Graphical representation of conventional mechanical ventilation (volume-controlled continuous mandatory ventilation [VC-CMV]) and airway pressure release ventilation (APRV) superimposed on a pressure-volume curve. To achieve the same mean lung volume, conventional ventilation, with its lower inspiratory-expiratory ratio, would require a higher PEEP, and thus, the delivery of the same tidal volume will have a higher end-inspiratory volume and hence an increased risk of injury by overdistension. From Reference 6, with permission.
APRV settings have also been analyzed in terms of how static versus dynamic strain determines lung injury.20,21 Strain is the ratio of VT to the functional residual capacity, and stress is the transpulmonary pressure change associated with the VT.20,21 Strain is composed of (1) dynamic strain, related to the cyclic inflation of the lung, and (2) static strain, the volume added to the functional residual capacity by the application of PEEP (or auto-PEEP). There is evidence that exceeding a strain threshold leads to ventilator-induced lung injury (VILI).22 Interestingly, it seems that the 2 types of strain are not equal in terms of the potential to induce VILI. Protti et al21 found that for a given amount of total strain, a higher component of static strain (PEEP, air trapping) leads to less lung injury than using dynamic strain. (ie, the combination of higher PEEP and lower VT is better than lower PEEP and higher VT). In theory, APRV allows the patient to spend most of the time in a situation of large static strain (Phigh) and small dynamic strain (spontaneous VT during Thigh) and thus should decrease the risk of lung injury compared with conventional modes that result in relatively low static and high dynamic strains. In a rat model of ARDS (not spontaneously breathing), Kollisch-Singule et al23 quantified the strain at a microscopic and macroscopic level. Indeed, APRV, when set with Tlow to achieve a terminal expiratory flow of ≥75% PEF, leads to the least amount of strain at both micro- and macroscopic levels. However, as the terminal expiratory flow decreased below 75% of PEF, the strain increased. The effect of spontaneous breaths has not been examined.
Roy et al24 compared conventional ventilation (10 mL/kg) versus APRV in pigs undergoing intestinal ischemia-reperfusion and peritoneal sepsis. Pigs were transitioned to low VT (6 mL/kg) when they met PaO2/FIO2 ARDS criteria. At 48 h, the APRV group had preserved lung architecture, better gas exchange, and lung compliance when compared with conventional ventilation. Similar findings have been seen in animal models of trauma and hemorrhagic shock.25 Emr et al26 demonstrated in a paralyzed rat model that conventional ventilation with VT of 10 mL/kg, when compared with APRV, led to histopathological changes and increased alveolar protein levels, which are features of acute lung injury. These studies support the concept that APRV may protect from development of acute lung injury. However, the challenge in interpreting these studies is that they did not use lung-protective strategies during conventional ventilation (large VT with a standard non-optimized PEEP) in paralyzed or sedated animals.
This technical feature serves the goal of safety by decreasing the risk of lung injury. For this to happen, the operator must set the ventilator appropriately, the ventilator must perform as expected, and it may be necessary for the ventilator to have automatic features to be able to adjust or alarm as the patient's condition changes.
The Rate of Mandatory Breaths Will Be Lower Than in Conventional Pressure or Volume Control Ventilation.
Total minute ventilation in APRV is the sum of spontaneous and mandatory minute ventilation components. APRV is built on the premise of allowing spontaneous breaths. The mandatory breaths in APRV (being a form of IMV) are intentionally set at a lower frequency (eg, 10 breaths/min) than for conventional modes. In the context of lung injury and injurious ventilator settings, decreasing cyclic stretch would seem intuitively better. This claim has not been directly studied in APRV, but it has been studied in animals receiving conventional ventilation.27–30 Conrad et al28 demonstrated a clear relationship between capillary permeability (a marker of VILI) and breathing frequency; however, this was only with large VT (20 mL/kg). They found no effect of frequency in the animals ventilated with VT of 5 mL/kg. Vaporidi et al27 and others also demonstrated a direct relation of breathing frequency with lung injury; however, this was ameliorated by decreasing VT. In summary, current basic animal research suggests that breathing frequency, in the setting on injurious ventilation, may cause further VILI. Thus, lower frequencies only serve the goal of safety (by minimizing lung injury), when the operator allows inappropriately high VT.
Auto-PEEP May Have Advantages Over Set PEEP to Preserve Lung Recruitment.
APRV uses short release (Tlow) to generate PEEP. There is some evidence to suggest that extrinsic PEEP is different compared with intrinsic PEEP. Both types of PEEP generate end-expiratory pressure; however, the way recruitment and de-recruitment happens may be different. Using a novel method of terminal airway analysis, Kollisch-Singule et al31 demonstrated that PEEP generated by the use of short expiratory times (APRV set with Tlow to keep terminal expiratory flow ≥75% of PEF) leads to gas distribution predominantly toward the alveoli rather than the conducting airways compared with conventional ventilation with a set PEEP of 16 cm H2O. Although interesting, this study may have been biased because it did not adjust the amount of extrinsic PEEP on conventional ventilation to mimic the auto-PEEP in APRV (ie, setting the PEEP at the same level as auto-PEEP). Nevertheless, the observations of Kollisch-Singule add information to the observations done by Neumann et al.32 They described the effects of pressure on the dynamics of lung recruitment and de-recruitment. In their animal model of lung injury, recruitment of the lung was incomplete even at high pressures applied over 4 s. During exhalation, de-recruitment occurred very rapidly; the time constant was calculated to be 0.69 ± 0.54 s, such that 85% de-recruitment occurred within 1.4 s. Consequently, to prevent de-recruitment, Tlow should be set ≤0.6 s in this model. In fact, in the study by Kollisch-Singule et al,31 the difference in Tlow to achieve terminal flow of 75% versus 10% of PEF was a mere 0.2 s (0.14 ± 0.01 s vs 0.34 ± 0.02 s). Not achieving this had a severe impact on alveolar recruitment and distribution of air.
This technical feature serves the goal of safety by minimizing lung injury, although for this to happen, the operator must set the ventilator appropriately. In summary, long inspiratory times favor recruitment, and very short expiratory times are needed to preserve the recruitment. The consequence of this is that most of the time is spent in Thigh, resulting in a low frequency of mandatory breaths, and the burden of minute ventilation has to be shifted to the patient's spontaneous breaths. These studies did not analyze the presence of spontaneous breathing.
Preservation of Spontaneous Breathing to Improve Gas Exchange.
The premise that makes APRV different from inverse ratio PC-continuous mandatory ventilation (CMV) is the presence of spontaneous breathing (ie, IMV vs CMV). Allowing the patients to breathe spontaneously (ie, patient-triggered and patient-cycled breaths) instead of imposing all mandatory breaths (patient- or machine-triggered and machine-cycled) alters the distribution of ventilation and perfusion.33 The rationale is that by allowing the diaphragm to remain active and contributing to the generation of a transpulmonary pressure gradient, the ventilation/perfusion ratio will be improved. The clinical evidence of the effect of spontaneous breathing was described in the 1990s34–36 and consistently demonstrates improved gas exchange due to improved ventilation/perfusion matching.
One must be careful when interpreting the effect of spontaneous breathing in APRV in the context of lung injury. Multiple factors interplay during the combination of spontaneous and mandatory breaths,37 ventilator settings,38 and degree of lung injury.39 Issues as simple as how the release time is set40 or the presence of automatic synchronization41 affect the outcome. This is highlighted by Neumann et al,42 who found that as the settings reflected more APRV (ie, inverse ratio), the VT size and variability decreased, and the amount of spontaneous breathing increased. Guldner et al43 examined what proportion of spontaneous ventilation to mandatory ventilation should be allowed and its effects in lung injury. In their animal model of ARDS ventilated with biphasic ventilation with an I:E of 1:1, most of the spontaneous breaths occurred in the Tlow. Finally, we must recognize that in APRV, the presence of spontaneous breathing and higher airway pressure may lead to injurious increases in transpulmonary pressure overall or regionally, thus leading to further lung injury. As a consequence, it is difficult to define what will be the effect on clinical practice.
This technical feature aims to serve the goal of safety by improving the gas exchange and perhaps to minimize lung injury. Research in this area needs to focus on best settings to allow spontaneous ventilation and on the effect of spontaneous breathing during Phigh.
APRV and Comfort
The technological features of the mode that may be of benefit in ARDS are described below.
Preservation of Spontaneous Breathing to Improve Patient-Ventilator Synchrony.
As mentioned above, a key feature of APRV is that it allows unrestricted spontaneous breathing. Some ventilator manufacturers have added the option to give pressure support or tube compensation to spontaneous breaths. Other ventilators have added synchronization windows to allow triggering and cycling of mandatory breaths to occur in synchrony with spontaneous breaths. The patient-ventilator interaction of APRV in the setting of ARDS was reviewed by Richard Kallet in this Journal.38 The intuitive thought is that allowing a patient to breathe spontaneously should be more comfortable and lead to less asynchrony. Unfortunately, the interaction between patient and APRV is not as simple as it may seem, which makes it harder to study. The spontaneous breaths may fall at any point during the ventilatory cycle; they may start synchronously with Thigh, during Thigh, synchronously at the end of Thigh, or anytime during Tlow (which is unlikely with very short Tlow settings)44 (Fig. 4). As a consequence, studies assessing the performance of APRV in terms of comfort, synchrony, and WOB need to define the amount and combination of breath types.43
Graphical representation of the locations where spontaneous breaths may occur during the airway pressure (Paw) release ventilation ventilatory cycle. A: Spontaneous breath during low CPAP. B: Spontaneous breath during high CPAP. C: Quasi-assisted breath that is sychronized with the ventilator cycling to the high CPAP level. D: Completely passive breath. E: Spontaneous breath that occurs as the ventilator cycles to the lower CPAP level. From Reference 38.
This technical feature aims to serve the goal of comfort by improving synchrony, However, at this point, we are unclear on the amount of spontaneous breathing that should be allowed or whether assisting and synchronizing breaths is appropriate.
WOB From the Patient May Be Decreased.
Another potential benefit of APRV is that spontaneous breathing leads to alveolar recruitment, improving the functional residual capacity and respiratory system compliance, leading to reduced elastic WOB. However, one must remember that in pressure control ventilation, the WOB performed by the ventilator in APRV is determined by the operator (ie, the pressure difference between Phigh and Plow and the rate) (Thigh and Tlow). The amount of work from the ventilator will not vary in relation to the patient WOB. APRV will not match rises in patient WOB; nor will it automatically decrease support in response to patient effort. Any increase or decrease of patient WOB will be left to the operator to detect and adjust the settings. Some ventilator manufacturers added the possibility of adding tube compensation or pressure support to assist spontaneous breaths; however, the effect of adding these features in the setting of ARDS is not defined.
APRV and Liberation
APRV allows the patient spontaneous breathing, which may have a beneficial effect on preserving diaphragm function. There are no automatic features in this mode that favor liberation because all titration of support is done by the clinician. Thus, all results will be heavily influenced by the practice and protocols used.
Summary of the Pro Position
APRV has technological features that serve the goals of safety and comfort. APRV has demonstrated in animal studies that it can promote alveolar stability and recruitment and minimize airway pressure, which seems to decrease or prevent lung injury. APRV also allows unrestricted spontaneous breathing; in our era of less sedation and increased mobility, this seems like a welcome feature; however, the amount of basic research on ARDS, APRV, and spontaneous breathing is scant. The history of ARDS highlights the importance of basic research in defining when and how to implement a mode. Modes of ventilation are just like medications; you need the correct one, at the correct dose, and for the right time for a given condition. APRV, like many other modes, is still looking for definition of the dose, the timing, and optimization of its delivery.
Con Position
Let us begin the con discussion by pointing out that most of the referenced evidence in the pro discussion focuses on animal models or lung model data. We will also make some reference to these types of studies, but the proof of benefit of a ventilatory technique needs to be evident in the patient literature. Yes, we can list numerous case series18,35 that imply that APRV is better than conventional mechanical ventilation, but considering that APRV has been available since 1986, there is surprisingly little data attempting to evaluate its performance compared with conventional mechanical ventilation in prospective randomized controlled trials (RCTs).34,45 In fact, there is only one RCT by Maxwell et al45 using the inverse ratio approach for the application of APRV.
The Maxwell study45 is a randomized comparison of APRV with conventional mechanical ventilation in a series of adult trauma subjects in acute respiratory failure. Conventional ventilation was applied using a low VT approach with volume-targeted SIMV and pressure support, and APRV was applied as discussed in the pro section with a very short Tlow. No differences in the demographics of the 2 groups could be identified. Gas exchange was equivalent between the 2 groups despite a significantly higher mean airway pressure in the APRV group (18–20 cm H2O vs 13–16 cm H2O in the conventional ventilation group over the first 5 d of mechanical ventilation). There was no difference in mortality (6.45% APRV and 6.25% control) or other complications, but there was a trend toward increased length of mechanical ventilation (10 ± 7.25 d APRV vs 8.00 ± 4.01 d control) and ICU stay (16.47 ± 12.83 d APRV vs 14.18 ± 13.26 d control) in the APRV group. However, of a total of 31 subjects in the APRV group, only 8 had ARDS and 6 had acute lung injury; similarly, in the control group, 9 of 32 had ARDS and 2 had acute lung injury. This is the single RCT that included some ARDS subjects from which we have to make the determination of whether APRV should be the primary mode in ARDS!
We would also caution against extrapolation of animal data to direct patient outcome. We have learned the hard way that what seems very promising in the animal laboratory and in case series frequently may actually be detrimental to patients. One just has to look at the RCTs on liquid ventilation46 and high-frequency oscillatory ventilation47,48 to illustrate that what is successful in the laboratory does not necessarily result in success at the bedside.
APRV and Safety
Higher Mean Airway Pressures for a Given Minute Ventilation and Peak Airway Pressure.
On the pro side, it was indicated that a higher mean airway pressure for a given minute ventilation and peak airway pressure results in better gas exchange. This is not necessarily true; when the lung is recruited and appropriate PEEP is applied, oxygenation is improved and does not necessarily require a higher mean airway pressure.49 In fact, the higher mean airway pressure may result in poorer oxygenation because of its effect on venous return and cardiac output. The results of the Maxwell study45 establish this point very nicely. Oxygenation was equivalent between the 2 groups despite mean airway pressure being higher in the APRV group.
Ventilation Occurs With Potentially Less Risk of VILI.
The data referenced to support this point on the pro side are from animal studies.23–25 However, one simply has to examine the esophageal pressure changes during APRV in actual patients to critically question this assumption. Figure 5 from Neumann et al42 illustrates the esophageal pressure changes required to breathe spontaneously at Phigh during APRV. As you will note, on the right-hand side of the figure, the approach to APRV is the one described in the pro side and the approach most commonly applied to patients with ARDS. Lung injury is ultimately caused by lung stretch (stress), and the best indicator of pulmonary stress is transpulmonary pressure (Ptp).50 We obtain Ptp by subtracting esophageal pressure (Pes) which is a reflection of pleural pressure from the plateau pressure (Pplat): Static Ptp = Pplat − Pes. In Fig. 5, the Pplat (Phigh during spontaneous inspiration) is ∼17 cm H2O, and the esophageal pressure change of ∼−13 cm H2O results in an end-inspiratory transpulmonary pressure of ∼30 cm H2O. If the Phigh level were higher, as it is frequently in the management of ARDS, closer to 30 cm H2O, the transpulmonary pressure would be well over 40 cm H2O, indicating a high probability of inducing lung injury. Based on information currently available, there are no patient data indicating a decreased likelihood of lung injury in APRV compared with conventional mechanical ventilation. In fact, based on expected transpulmonary pressure swings during spontaneous breathing at Phigh, the likelihood of lung injury is greater with APRV than with conventional mechanical ventilation with VT of ∼6 mL/kg predicted body weight.51
Airway pressure (Paw), flow, volume, and esophageal pressure (Pes) during airway pressure release ventilation (APRV). A: APRV with a 1:1 ratio between Phigh and Plow. B: The idealized approach to APRV with a short (<1-s) Tlow. Note the large swings in esophageal pressure as both patients attempt to breathe spontaneously at Phigh and Plow. From Reference 42, with permission.
The other factor that must be considered when evaluating potential for lung injury is the size of the VT produced with the change from Plow to Phigh. When documented, this VT appears to be consistently higher than the 4–8 mL/kg predicted body weight range currently recommended, not only for ARDS patients but for all patients acutely requiring ventilatory support.18,42 In some cases, the VT change from Plow to Phigh has been measured at >10 or 11 mL/kg predicted body weight, clearly not lung-protective in ARDS patients. The greater the difference between Plow and Phigh, the greater will be the VT, and current recommendations can set the Plow as low as 0 cm H2O.
The Rate of Mandatory Breaths Will Be Lower Than in Conventional Pressure or Volume Control Ventilation.
Yes, the mandatory breath frequency is generally lower in APRV than in conventional ventilation. However, there are no data available to indicate that when conventional mechanical ventilation is provided in an appropriate lung-protective strategy, as it is today in the management of ARDS, then breathing frequencies into the 30s result in any adverse outcome (as long as the rapid frequency does not cause air trapping). In fact, if one reviews the many positive lung-protective ventilation RCTs, the respiratory rate in the low VT groups was always higher than that in the control group with beneficial effects on outcome.51–54
Auto-PEEP May Have Advantages Over Set PEEP to Preserve Lung Recruitment.
There are no patient data to indicate that this is true. Auto-PEEP is established primarily in lung units with long expiratory time constants.55 To have a long expiratory time constant, lung compliance must be increased and/or airway resistance must be increased. In ARDS, the lung units in which PEEP is needed are the lung units with the shortest expiratory time constants; those with lengthy expiratory time constants are either without disease or minimally diseased. When time constants are calculated (resistance time compliance = time constant) in any patient or model, the calculation reflects the average global time constant of the lung in question. Thus, no matter what the calculated time constant, the most diseased lung units in ARDS will have a shorter time constant. As a result, even with Tlow as short as 0.5–0.7 s, some lung units will de-recruit, and those that de-recruit will be lung units that have the lowest compliance, the lung units most in need of PEEP. This concept is easily demonstrated in a simple lung model56 (see Fig. 6). The establishment of set PEEP in Figure 6 by the ventilator results in PEEP being applied equivalently to all lung units regardless of time constants, and it is not lost during the expiratory phase. It is impossible for auto-PEEP to maintain better lung recruitment than applied PEEP because auto-PEEP is distributed primarily to lung units with long time constants, and during the Plow phase of APRV, regardless of the Tlow, volume will be lost from those lung units with the shortest expiratory time constants.
Airway versus time waveforms in a 4-chamber lung model with different lung unit time constants during the application of a set PEEP of 12 cm H2O (A) and the establishment of 12-cm H2O auto-PEEP (B). The lung model has one slow time constant lung unit, one fast time constant lung unit, and 2 normal time constant lung units. Note that with applied PEEP, the PEEP level in all lung units was constant at 12 cm H2O. However, with auto-PEEP, the fast time constant lung unit PEEP level was lower than the normal time constant lung units, and the slow time constant lung unit PEEP was higher than the normal lung units. From Reference 56, with permission.
Preservation of Spontaneous Breathing to Improve Gas Exchange.
All would agree that maintaining spontaneous breathing over sedation/paralysis and controlled ventilation is preferable and this fact has been clearly demonstrated in patients.34,35 However, patient triggering of the ventilator has increasingly become the norm for conventional mechanical ventilation of patients with ARDS unless patients have severe ARDS (PaO2/FIO2 <100). As has been clearly demonstrated by Papazian et al,57 patients with severe ARDS have lower mortality when they are paralyzed and sedated for the first 48 h of mechanical ventilation than when they only receive sedation.
Patient triggering of ventilation does occur in volume control-CMV, PC-CMV, and PC-continuous spontaneous ventilation. Although this may not result in the same global effect as unsupported spontaneous breathing, as seen in APRV, patients are allowed to interact with the ventilator, improving gas distribution. The spontaneous breathing associated with APRV at high Phigh levels has not been shown to result in better patient outcome than the patient triggering of assisted breaths in PC-CMV, PC-CMV, or PC-continuous spontaneous ventilation and does require excessive patient effort, as described in the next section. Again, note that in the Maxwell study,45 there were no differences in oxygenation, ventilation, or acid/base balance between the APRV group and the conventional mechanical ventilation group.
APRV and Comfort
Preservation of Spontaneous Breathing to Improve Patient-Ventilator Synchrony.
As discussed in the pro section, spontaneous breathing in APRV is not as straightforward as spontaneous breathing in simple CPAP.38,43,44 During CPAP, there is no question that synchrony is better than with any other mode of ventilation because the only interaction required is a greater opening of the demand flow system, and if the ventilator is capable of meeting the patient's inspiratory demand, asynchrony should be minimal. However, during APRV, the unsynchronized transition from Phigh to Plow and from Plow to Phigh can create very asynchronous breaths if the patient is in the opposite phase of ventilation when this transition occurs.38,42,44 In addition, the cost of breathing spontaneously during ARPV is markedly elevated42,44 (Fig. 5). For patients to inspire spontaneously at Phigh, they had to create a 13-cm H2O pleural pressure gradient, clearly excessive for any patient requiring ventilatory support. If patients receiving APRV are more comfortable than they are receiving conventional mechanical ventilation, then why in the Maxwell study45 did subjects receiving APRV clearly have a trend of requiring more sedation and narcotics than subjects in the control group (Fig. 7)?
Fentenyl and lorazepam use for the first 5 d of ventilatory support are presented. The large SD values prevented significant differences between airway pressure release ventilation (APRV) and conventional mechanical ventilation, but a strong trend indicates less use of these drugs in conventional mechanical ventilation as compared with airway pressure release ventilation. From Reference 45, with permission.
WOB From the Patient May Be Decreased.
On the contrary, the WOB during APRV can be expected to be markedly elevated compared with other conventional modes of ventilation. Figure 5 clearly illustrates this point.42 If patients are required to inspire spontaneously without any ventilatory assistance at high Phigh levels, then large transpulmonary pressure swings can be expected (as illustrated in the esophageal pressure tracing in Fig. 5). Patients with ARDS are very critically ill, and we provide ventilatory assistance to relieve the WOB and the work of the cardiovascular system and to improve gas exchange while the patient recovers. Forcing patients to breathe at Phigh levels that require high pleural pressure swings does not reduce the WOB. Clearly, the greater the lung injury/greater the decrease in lung compliance, the greater will be the unassisted WOB during APRV.
APRV and Liberation
There are no data whatsoever to imply that patients are liberated from the ventilator faster with APRV. In fact, in the study by Maxwell et al,45 there was a trend toward longer length of mechanical ventilation and ICU stay in patients maintained with APRV than in those with conventional mechanical ventilation.
Summary of the Con Position
APRV does not reduce the WOB, APRV does not reduce the need for sedation and narcotics, APRV does not minimize the likelihood of VILI, APRV does not reduce the time required for mechanical ventilation or ICU stay, and APRV does not affect mortality. APRV does have a greater potential for adversely affecting patient outcome than improving it, and unless definitive data are forthcoming demonstrating outcome benefits from the use of APRV in ARDS or any other patient group, there is no reason to consider this approach to ventilatory support. This conclusion is consistent with that expressed in other reviews of APRV.38,58,59
Discussion
MacIntyre:
How many people around the room use APRV anything other than once in a blue moon? Anybody? Let the record show that nobody raised his hand.
Marini:
Very nice, Bob. There are 2 physiological aspects of APRV that might be interesting to bring up. One relates APRV to the current focus is on driving pressure as a risk factor for VILI. Driving pressure is probably a more interesting variable than VT, for sure. Just concentrating on the airway pressure swing associated with the release cycles of APRV, the driving pressure during the inflation phase is usually very high. That would complement your worry that you might be damaging the lung. On the opposite side, we worry about HFOV [high-frequency oscillatory ventilation] and its hemodynamic effects when it's used at high levels as it was in the OSCILLATE Trial.1 Interestingly enough, there isn't much hemodynamic impact of APRV at a similar ̄Paw. So to sum this up, when people say why did OSCILLATE fail, they point to hemodynamic reasons. But if you compare the ̄Paw associated with HFOV and the ̄Paw associated with APRV, you're talking about a similar range. Why are we not seeing reports of adverse hemodynamic consequences with APRV?
Kacmarek:
I think it's the fact that patients are spontaneously breathing, and we have this huge intrathoracic pressure change facilitating venous return.
Marini:
Not always—they don't always spontaneously breathe.
Kacmarek:
Well, it's a different discussion if we get into patients in controlled mechanical ventilation during APRV compared to patients who are breathing spontaneously. I focused on the potential injury due to the fact that they are breathing spontaneously. If they are totally sedated, then I would agree with you, the ̄Paw values are higher, but most of the data on APRV focuses on spontaneous ventilation as opposed to sedation and paralysis.
Mireles-Cabodevila:
I would add that another difference with OSCILLATE was that it was a systematic collection of data under controlled conditions. In APRV, we don't have any trial with a large number of subjects and under controlled circumstances.
Marini:
There's another interesting possibility: The right ventricle is thought to be one of the real problems, hemodynamically, in ARDS and ventilator support.
Kacmarek:
Agreed.
Marini:
Let's say somebody's gently breathing on APRV. They're not vigorously breathing, and you have these release cycles going on. With HFOV, high airway pressure is unrelieved, and so is the associated increase in RV (right ventricle) afterload, whereas in APRV, there's periodically a transient release. And I wonder if that isn't part of the issue. Do you have an opinion?
Kacmarek:
As we've all said, I don't have much experience with APRV, so it's difficult to speak from a clinical perspective.
Marini:
That's never deterred you before!
Kacmarek:
Just like your data,2 John, with lung recruitment maneuvers; you do a static recruitment maneuver, you have clearly more impact on hemodynamics than if you keep the same peak pressure, but you go and ventilate the patient even if you do it at a relatively slow pace. That decrease of pressure even for a short period of time seems to improve the hemodynamic status. So, you may well be right that release of pressure, in some of these case series, to very low levels improves hemodynamics. Some set low PEEP at 0, so there's a huge release of pressure and potentially rapid change in the hemodynamic status.
Mireles-Cabodevila:
The paper that makes this more relevant was the Yoshida paper3 in Brazil, where they describe occult pendelluft in the lung and localized increases in Ptp in certain areas of the lung. The relevance is that it's not only what we see at the esophageal level; regional Ptp can be very elevated and, in the presence of higher ̄Paw (as in APRV), can be injurious.
Kallet:
A few other observations and commentary. First, APRV is the devil's spawn! I'll let that hyperbole be published. I have actually measured WOB in APRV.4 In part, I did this because shortly after publication of the ARDS Net study,5 Drs Brower, MacIntyre and I presented a mini-symposium on ARDS Net at the AARC Congress. There was a proponent of APRV speaking at the same congress trying to convince our colleagues that you could get the same results by using APRV with the ARDS Net protocol. So I tried to mimic that. The first problem you encounter is that if you use APRV in severe ARDS, as described in the literature, your release volumes are 800-900 mL. So, yes, in someone who is dyspneic and short of breath, you can control their spontaneous WOB by diminishing dyspnea and decreasing respiratory drive, but what are you actually doing in terms of patient outcomes? When you try, as I tried to do, to lower your release volume to about 7 mL/kg for lung-protective ventilation, you have these enormous increases in negative intrathoracic pressures. It was actually difficult to safely collect the data on this. But this brings up the point about the wisdom of allowing spontaneous breathing in someone who's in severe respiratory distress regardless of mode choice. When you're in respiratory distress, you are enhancing pulmonary edema; you're causing more fluid transudation into the lungs because of increased negative intrathoracic pressure swings.6 At the same time, the natural reaction when WOB is severe (or the work of any skeletal muscle for that matter) is to reflexively try to get maximal relaxation, so the next contraction is more forceful. The way that happens in the respiratory system obviously is to recruit the abdominal muscles and punch the diaphragm up into the chest, therefore counteracting the effectiveness PEEP. In other words, at the same time that spontaneous breathing exacerbates pulmonary edema, it also reduces alveolar stability and impairs oxygenation. I think this is one of the main reasons that paralysis works in severe ARDS. You really do not want someone interacting with a ventilator when they're in severe distress and they have an injured alveolar capillary membrane.
Marini:
Exactly right. I'm not surprised that I agree with you, Rich, but there's a big difference between gently breathing and vigorously breathing. It's an important point.
Branson:
I just want to say, having put people on APRV when a CPAP generator, 2 CPAP valves, and a solenoid was used to switch back and forth, almost all this work was done in surgical or trauma patients. And the reason they tolerate it hemodynamically is because they fluid-load them. At SCCM [Society of Critical Care Medicine] this past year, an abstract was presented demonstrating all the weaning data from a single university7 from every ICU. The one ICU that routinely uses APRV had the longest length of mechanical ventilation in the hospital, and it was 30-40% greater than the other ICUs. I firmly believe that it's because it takes the patients who long to mobilize the fluid until they're able to come off the ventilator.
Kallet:
I think that's part of it too. Another aspect becomes apparent when you look at their protocol in terms of weaning. Ostensibly, you slowly decrease these cycling drops to Tlow until you're at one CPAP level, and only then do you bring that level down. It's like IMV; it artificially increases weaning time just because of how you designed the protocol.
Branson:
My favorite story about APRV is when it was introduced to the FDA, the FDA had a rule that inspiratory time could never be longer than 4 times the expiratory time. So in order to get this method cleared, they had to come up with a name (APRV), and they had to call it Thigh and Tlow and Phigh and Plow in order to get it through the FDA. I've always been frustrated why didn't they just call it Ppeak? It's a version of IMV, and it was because they couldn't get it through the FDA unless they changed the terminology.
Footnotes
- Correspondence: Eduardo Mireles-Cabodevila MD, Respiratory Institute, Cleveland Clinic, 9500 Euclid Avenue, A90, Cleveland, OH 44195. E-mail: mirelee{at}ccf.org. Robert M Kacmarek RRT PhD FAARC, Respiratory Care, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 01460. E-mail: rkacmarek{at}partners.org.
Drs Mireles-Cabodevila and Kacmarek presented a version of this paper at the 54th Respiratory Care Journal Conference, “Respiratory Care Controversies III,” held June 5–6, 2015, in St Petersburg, Florida.
Dr Kacmarek has disclosed relationships with Covidien, Orange Medical, and Venner Medical. Dr Mireles-Cabodevila has no conflicts to disclose.
- Copyright © 2016 by Daedalus Enterprises