Abstract
BACKGROUND: Critically ill patients with respiratory failure undergoing intubation have an increased risk of hypoxemia-related complications. Delivering oxygen via a high-flow nasal cannula (HFNC) has theoretical advantages and is increasingly used. This study was conducted to compare HFNC with bag-valve-mask (BVM) for preoxygenation and to assess oxygenation during intubation in subjects with hypoxemic respiratory failure.
METHODS: This study was a randomized controlled trial including 40 critically ill subjects with hypoxemic respiratory failure who received either HFNC or BVM for preoxygenation before intubation in the ICU. The primary outcome was the mean lowest SpO2 during intubation.
RESULTS: The mean lowest SpO2 during intubation was 89 ± 18% in the HFNC group and 86 ± 11% in the BVM group (P = .56). In subjects receiving HFNC, a significant increase in SpO2 after preoxygenation was only seen in those previously receiving low-flow oxygen (P = .007), whereas there was no significant difference in SpO2 in subjects previously receiving noninvasive ventilation or HFNC (P = .73). During the 1 min of apnea after the induction of anesthesia, SpO2 dropped significantly in the BVM group (P = .001), whereas there was no significant decrease in the HFNC group (P = .17). There were no significant differences between the 2 groups at any of the predefined time points before or after intubation concerning SpO2, PaO2/FIO2, and PaCO2.
CONCLUSIONS: Preoxygenation using HFNC before intubation was feasible and safe compared with BVM in critically ill subjects with acute, mild to moderate hypoxemic respiratory failure. There was no significant difference in the mean lowest SpO2 during intubation between the HFNC and the BVM group. There was also no significant difference in SpO2 between the 2 groups at any of the predefined time points. However, on continuous monitoring, there was a significant decrease in SpO2 during the apnea phase before intubation in the BVM group, which was not seen in the HFNC group. (ClinicalTrials.gov registration NCT01994928.)
Introduction
In contrast to patients undergoing scheduled endotracheal intubation for elective surgery, critically ill patients in the ICU, especially those with acute hypoxemic respiratory failure, are at increased risk for severe life-threatening complications.1–4 A study on 253 endotracheal intubations in ICU subjects documented the occurrence of severe complications in 28% of cases, with severe hypoxemia accounting for 26% of these events. Acute respiratory failure was shown to be an independent risk factor for the occurrence of complications.5 Another study including 136 ICU subjects showed an overall risk for complications of 39% with severe hypoxemia being the most common complication.1 A study by Mort6 including 42 critically ill subjects concluded that, in this population, preoxygenation using a bag-valve-mask (BVM) was only marginally effective for preventing hypoxemia during endotracheal intubation. Hypoxemia followed by hemodynamic deterioration and cardiac arrest is the most common cause of airway-related death.2,7 Therefore, great care is taken to minimize risks by adhering to standardized protocols including preoxygenation.8 Different techniques for preoxygenation, including the use of a BVM6 or noninvasive ventilation (NIV),9 have been investigated in critically ill subjects requiring endotracheal intubation.
High-flow nasal cannula (HFNC) oxygen is increasingly applied in adult ICU patients with acute hypoxemic respiratory failure as an alternative to NIV.10 HFNC delivers heated and humidified gases at a flow of up to 60 L/min via a nasal cannula, providing a high level of oxygen covering the inspiratory flow and allowing setting of inspiratory FIO2. This results in effective and sustained improvement in respiratory parameters in patients with acute hypoxemic respiratory failure by several mechanisms, especially the generation of PEEP, pharyngeal dead space washout, and the reduction of nasopharyngeal resistance.11 Apneic oxygenation (ie, delivering oxygen to the airways and lungs without ventilation) has been shown to prolong the time to hypoxemia in subjects with healthy lungs12 and in a model of acute lung injury.13 In contrast to other techniques, the nasal cannulas for high-flow oxygen delivery do not interfere with laryngoscopy and therefore can be used to deliver oxygen during the apneic period of endotracheal intubation.11
To our knowledge, until now, only 2 studies have evaluated the effectiveness of HFNC for preoxygenation before endotracheal intubation in critically ill subjects with acute respiratory failure. The only randomized trial was published by Vourc'h et al,14 comparing HFNC with high FIO2 applied via face mask. The other study was published by Miguel-Montanes et al15 and compared preoxygenation using a facial mask with HFNC in a before-and-after study. We therefore conducted this randomized trial comparing HFNC with BVM for preoxygenation and assessing oxygenation during intubation in subjects with acute hypoxemic respiratory failure.
QUICK LOOK
Current knowledge
Critically ill patients with respiratory failure undergoing intubation have an increased risk of hypoxemia-related complications. Delivery of oxygen via a high-flow nasal cannula (HFNC) is increasingly used and has theoretical advantages. However, the value of HFNC for preoxygenation in patients with hypoxemic respiratory failure has not been thoroughly investigated.
What this paper contributes to our knowledge
Preoxygenation using HFNC before intubation was feasible and safe compared with bag-valve-mask (BVM) in critically ill subjects with mild to moderate hypoxemic respiratory failure. There was no significant difference in the mean lowest SpO2 during intubation between the HFNC and BVM groups. A significant improvement in SpO2 after preoxygenation using HFNC was only seen in subjects who were previously receiving low-flow oxygen, whereas there was no significant difference in SpO2 in subjects previously receiving NIV or HFNC. In contrast to the BVM group, there was no significant decrease in SpO2 during the apnea phase before intubation in the HFNC group.
Methods
Study Design
This was a prospective randomized trial. All adult patients admitted to the Department of Intensive Care Medicine at the University Medical Center Hamburg-Eppendorf were eligible for study inclusion. Before enrollment, all participants or their legal representatives gave written informed consent. The study was performed in accordance with the Declaration of Helsinki. It was approved by the ethics committee of the Hamburg Chamber of Physicians (reference number PV4429, date of approval November 4, 2013) and registered at ClinicalTrials.gov (registration number NCT01994928, registration date November 20, 2013).
Study Population
Medical and surgical patients treated in any of the 11 departmental ICUs were enrolled. Inclusion criteria were: (1) respiratory failure with hypoxemia defined as PaO2/FIO2 <300 mm Hg, (2) indication for endotracheal intubation, (3) age ≥18 y, and (4) informed consent. Exclusion criteria were: (1) contraindications for BVM or HFNC, (2) nasopharyngeal obstruction or blockage, (3) emergency endotracheal intubation, and (4) suspected or known difficult airway (Mallampati class >2, reduced neck movement, reduced mouth opening, or Cormack-Lehane grade 4 recorded during a previous intubation procedure).
Study Protocol
After enrollment, subjects were randomized to receive preoxygenation using either HFNC or BVM. Randomization was accomplished by computer-generated random number sequence allocation, which was concealed from the study team by using numbered, opaque, and sealed envelopes.
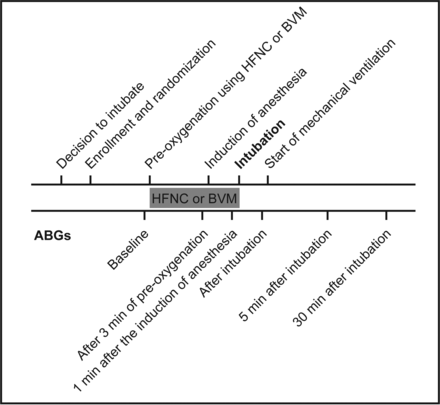
Arterial blood gases were drawn from an indwelling catheter in the radial or femoral artery. In our ICU, an indwelling arterial catheter is routinely placed in patients with respiratory failure and before intubation. Blood samples were analyzed immediately using an analysis machine (Radiometer ABL90, Radiometer Medical ApS, Brønshøj, Denmark) in the ICU. Arterial blood gases were collected at predefined time points: baseline, after 3 min of preoxygenation using BVM or HFNC, 1 min after the induction of anesthesia just before intubation, immediately after intubation just before the start of mechanical ventilation, and 5 and 30 min after intubation. FIO2 was also recorded. In subjects receiving low-flow oxygen therapy at baseline, a conversion table (Table 1) depending on the device and the oxygen flow was used to estimate the FIO2 delivered to the subject. SpO2, blood pressure, heart rate, and breathing frequency were monitored constantly throughout this period. The lowest SpO2 observed while being monitored during intubation was registered. For details on study workflow, see the flow diagram in Figure 1.
Conversion Table for FIO2 with Low-Flow Oxygen
Study workflow. ABG = arterial blood gas analysis; HFNC = high-flow nasal cannula oxygen therapy; BVM = bag-valve-mask ventilation.
Bag-Valve-Mask
For preoxygenation using a BVM, an adult size AMBU SPUR II disposable resuscitator with oxygen bag reservoir and without PEEP valve or pressure manometer (AMBU, Bad Nauheim, Germany) was used. Oxygen flow was set to 10 L/min. During preoxygenation, the breathing frequency was defined by the subject's spontaneous breathing. No manual insufflations were performed during the apneic period. The BVM was removed immediately before intubation to allow this procedure.
High-Flow Nasal Cannula Oxygen
For preoxygenation using HFNC, an Optiflow system with a medium size adult nasal cannula as patient interface (Fisher and Paykel Healthcare Ltd, Auckland, New Zealand) was used in all cases. The system includes a blender, allowing the selection of an FIO2 between 0.21 and 1.0, and routes the gas through a chamber where it is heated and humidified before being delivered to the patient. Oxygen flow was set to 50 L/min, and the FIO2 was set to 1.0. The HFNC was left in place during the intubation procedure.
Endotracheal Intubation
The decision to intubate was left to the discretion of the intensivist in charge of the subject in accordance with published guidelines.16 In our department, endotracheal intubation and patient management are conducted according to standardized protocols adopted by all of the departmental ICUs. After preoxygenation, a rapid sequence induction using sufentanil, propofol, and rocuronium was performed according to the local protocol. Endotracheal intubation was monitored by direct laryngoscopy. After placement of the endotracheal tube, the subject was connected to the ventilator (Evita Infinity V500, Dräger, Germany). The ventilator was initially set to pressure controlled mode and an FIO2 of 1.0. The settings were then adapted to achieve adequate oxygenation and ventilation. Correct tube placement was verified by visual inspection of chest movements, auscultation, and capnography in all cases.
Outcome Parameters
The primary outcome parameter was the mean lowest SpO2 during intubation. Secondary outcome parameters were the changes in arterial blood gases up to 30 min after intubation. Adverse events related to the intubation were recorded. Adverse events were defined as death; cardiac arrest; arrhythmias; hemodynamic instability; aspiration of gastric content; or injury to the teeth, soft tissue, laryngeal structures, or tracheal wall. Sample size was calculated to allow the detection of a 3% difference in minimal oxygen saturation, assuming an α risk of .05 and a power of 0.8.
Statistical Analysis
Results are presented as absolute numbers and percentages and as mean ± SD for normally distributed continuous data. Comparison between the 2 groups was performed using the t test for metric data and the chi-square test for categorical data. A 2-sided P value of <.05 was considered significant. The software used for descriptive analyses was SPSS 20.0 (SPSS, Chicago, Illinois).
Results
Subject Characteristics
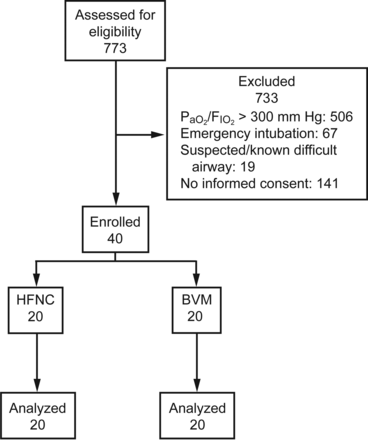
Between January and November 2014, 40 subjects were included after obtaining informed consent and randomized to receive preoxygenation using either HFNC or BVM. The subject flow diagram of the study is shown in Figure 2. Most subjects were surgical patients (95% in the BVM group and 75% in the HFNC group). Hospitalization was most often due to malignant or non-malignant conditions of the gastrointestinal tract or abdomen, followed by vascular diseases and community-acquired pneumonia. Mean PaO2/FIO2 at baseline was 200 ± 57 mm Hg in the HFNC group and 205 ± 59 mm Hg in the BVM group (P = .76). Mean SpO2 at baseline was 96 ± 3% in the HFNC group and 94 ± 4% in the BVM group (P = .24). There were no significant differences between the 2 groups for respiratory rate, heart rate, or mean arterial pressure at baseline. Table 2 provides further details on the participants' characteristics.
Flow chart. BVM = bag-valve-mask ventilation; HFNC = high-flow nasal cannula oxygen therapy.
Characteristics of Subjects
Intubation Procedure
Board-certified intensivists performed all intubations. There were no significant differences between the 2 groups with regard to the laryngoscopic view (Cormack-Lehane grading); the duration of intubation; the amounts of sufentanil, propofol, or rocuronium used; or the initial PEEP just after intubation. Table 3 provides details on the intubation procedure.
Characteristics of the Intubation Procedure
Tolerance of the Procedure
The mean lowest SpO2 during intubation was 89 ± 18% in the HFNC group and 86 ± 11% in the BVM group (P = .56). After preoxygenation, SpO2 had significantly improved from baseline in the BVM group (from 94 ± 4 to 98 ± 4%, P = .004). In subjects receiving HFNC, a significant increase in SpO2 after preoxygenation was only seen in the subgroup who were previously receiving low-flow oxygen (from 95 ± 2 to 99 ± 3%, P = .007), whereas there was no significant difference in SpO2 in subjects previously receiving NIV or HFNC (P = .73). However, during the first minute of apnea after the induction of anesthesia, SpO2 dropped significantly in the BVM group (P = .001), whereas there was no significant change in the HFNC group (P = .17). Five subjects (25%) had an SpO2 <80% during intubation in both the HFNC group and the BVM group. Abortion of the preoxygenation or the apnea phase and emergency intubation due to rapid progressive hypoxemia were required in 2 cases each in the HFNC and the BVM groups. Subjects requiring emergency intubation were not excluded from the analysis.
There were no significant differences between the 2 groups at any of the predefined time points before and after intubation concerning SpO2, PaO2/FIO2, and PaCO2. As expected, during apnea, there was a significant and comparable increase in PaCO2 in both groups. Changes in SpO2 are shown in Figure 3. Changes in PaO2/FIO2 and PaCO2 before and after intubation are shown in Figure 4. There were no adverse events related to intubation.
SpO2 at baseline, pre- and post-intubation. Data are shown as mean ± SD. Pre-ox = after 3 min of preoxygenation; Pre = 1 min after the induction of anesthesia, before intubation; Post = immediately after intubation, before mechanical ventilation; HFNC = high-flow nasal cannula oxygen therapy; BVM = bag-valve-mask ventilation. *, significant change between time points within group (P < .05).
Changes in PaO2/FIO2 (A) and PaCO2 (B). An FIO2 of 1.0 was used to calculate PaO2/FIO2 at the end of the apneic period just before intubation. Pre-ox = after 3 min of preoxygenation; Pre = 1 min after the induction of anesthesia, before intubation; Post = immediately after intubation, before mechanical ventilation; HFNC = high-flow nasal cannula oxygen therapy; BVM = bag-valve-mask ventilation. *, significant change between time points within group (P < .05).
Discussion
In this randomized controlled trial, we compared the use of HFNC with BVM for preoxygenation before intubation and assessed oxygenation during intubation in critically ill subjects with acute hypoxemic respiratory failure. There was no significant difference in the primary outcome parameter, the mean lowest SpO2 during intubation, between the HFNC group and the BVM group. A significant improvement in SpO2 after preoxygenation using HFNC was only seen in the subgroup of subjects previously receiving low-flow oxygen, whereas there was no significant difference in SpO2 in subjects previously receiving NIV or HFNC. In contrast to the BVM group, there was no significant decrease in SpO2 during the apnea phase before intubation in the HFNC group.
The usefulness of HFNC as a means of preoxygenation before intubation has been discussed in theory.11,17 The potential advantage of HFNC in this specific situation is the possibility to maintain oxygen delivery during laryngoscopy. In an experimental model of respiratory failure, the pharyngeal administration of oxygen at a flow of 10 L/min delayed the time to severe desaturation during apnea.13 With all other preoxygenation devices, the mask has to be removed during laryngoscopy, which deprives the patient of oxygen during the actual intubation procedure. This may explain the significant desaturation in the BVM group of our study and highlights the advantage of HFNC and its uninterrupted oxygen delivery. However, even small decreases in oxygen saturation will, when approaching an SpO2 of 90%, lead onto the steep section of the oxyhemoglobin dissociation curve, where SpO2 will decrease rapidly to critical levels.
We observed a significant increase in SpO2 after preoxygenation using HFNC only in subjects previously receiving low-flow oxygen, and we saw no significant difference in SpO2 in subjects previously receiving NIV or HFNC. This may be due to the increase in disposable inspiratory oxygen provided by HFNC compared with low-flow oxygen. An alternative or additional explanation for this may be that subjects previously receiving low-flow oxygen were newly exposed to PEEP generated by HFNC after its initiation, whereas there was no additional PEEP effect in subjects already receiving HFNC or NIV. Positive airway pressures generated by HFNC are known to depend on oxygen flow and whether the person's mouth is open or closed. A mean positive airway pressure of 2.7 cm H2O has been reported with mouth closed, whereas only 1.2 cm H2O has been observed with mouth open.18 Therefore, closure of the mouth seems essential to maximize the effects of HFNC during preoxygenation. However, as discussed previously for the use of HFNC during flexible bronchoscopy in subjects with hypoxemic respiratory failure,19 patients with more severe respiratory distress frequently have a preference or need to breathe through an open mouth, which leads not only to a decrease in FIO2 but also to the absence of relevant positive airway pressure levels. Therefore, patients with severe hypoxemia should probably not undergo interventions using HFNC.20 However, our study was neither designed nor powered to answer this question. Further studies including more subjects are needed to assess this topic.
To date, only one other randomized study14 has compared HFNC with high FIO2 applied via face mask for preoxygenation in subjects with acute hypoxemic respiratory failure. Vourc'h et al14 found no difference between the 2 groups with regard to the number of ventilator-free days; intubation-related adverse events, including desaturation to an SpO2 of <80%; or mortality. However, this study used a face mask, which may deliver different FIO2 and achieve different oxygen levels during preoxygenation from those of the bag-valve-mask used in our study.21,22 Another difference from our study was the fact that subjects in the study by Vourc'h et al14 had a higher degree of hypoxemia (PaO2/FIO2 at baseline was 120.2 ± 55.7 mm Hg in the HFNC group and 115.6 ± 63 mm Hg in the face-mask group compared with 200 ± 58 mm Hg in the HFNC group and 205 ± 59 mm Hg in the BVM group in our study). Nevertheless, results were similar, with no obvious benefit for HFNC regarding preoxygenation in these subjects. Another study by Miguel-Montanes et al15 evaluated the use of HFNC for preoxygenation before intubation in critically ill subjects in a before-and-after study. The authors compared preoxygenation using a facial mask with an oxygen flow of 15 L/min for 3 min, followed by oxygen administered at a flow rate of 6 L/min via a nasopharyngeal catheter during apnea with preoxygenation using HFNC with an oxygen flow of 60 L/min.15 They found that HFNC significantly improved preoxygenation and reduced the incidence of severe desaturation. These results stand in contrast to the results of our study and that of Vourc'h et al.14 Possible reasons for the different findings are that in the study by Miguel-Montanes et al,15 subjects had only mild hypoxemia, illustrated by the fact that none had an SpO2 below 98% at baseline. Furthermore, patients already receiving HFNC or NIV were excluded from the study. In contrast, 28% of subjects in our study and 27% of subjects in the study by Vourc'h et al14 were receiving NIV or HFNC at baseline. As described above for preoxygenation using HFNC, the results of our study showed no significant increase in SpO2 after preoxygenation in subjects already receiving NIV or HFNC. Moreover, the study by Miguel-Montanes et al15 should be interpreted with caution because it used a before-and-after design, and arterial blood gases were only collected when available.23
The use of NIV for preoxygenation has been evaluated in a prospective randomized study comparing it with preoxygenation using a BVM.9 The authors found that NIV was more effective in reducing desaturation than BVM. Given the increasing use of HFNC in patients with hypoxemic respiratory failure, it would be interesting to compare HFNC and NIV for preoxygenation before intubation. Preoxygenation using HFNC without the need for transitioning to NIV might facilitate the procedure for ICU staff and provide improved patient comfort due to the avoidance of the potentially unpleasant experience of NIV.
Although HFNC has theoretical advantages and positive effects have been demonstrated in observational studies in subjects with ARDS,24 several aspects regarding the outcome of this intervention are currently unknown. Kang et al25 investigated 175 subjects with HFNC and questioned whether the failure of HFNC may have caused a delayed intubation and worsened clinical outcome in subjects with acute hypoxemic respiratory failure. Therefore, we believe that close monitoring of patients receiving HFNC is mandatory.
Our study has certain limitations. We performed preoxygenation using the BVM with an oxygen flow of 10 L/min and without a PEEP valve. It is possible that higher oxygen flow and the application of a PEEP valve might improve preoxygenation using a BVM. The results may not be generalized to different populations or settings. The subjects in our study had high Simplified Acute Physiology Score II, and a quarter of them required intubation due to progressive hypoxemia. Furthermore, all intubations were performed in the ICU by experienced intensivists following a standardized departmental protocol.
Conclusions
Preoxygenation using HFNC before intubation was feasible and safe compared with BVM in critically ill subjects with mild to moderate hypoxemic respiratory failure. In fact, we found no significant difference in the mean lowest SpO2 during intubation between the 2 groups. A significant improvement in SpO2 after preoxygenation using HFNC was only seen in the subgroup of subjects previously receiving low-flow oxygen, whereas there was no significant difference in SpO2 in subjects previously receiving NIV or HFNC. In contrast to the BVM group, there was no significant decrease in SpO2 during the apnea phase before intubation in the HFNC group.
Footnotes
- Correspondence: Stefan Kluge MD, Department of Intensive Care Medicine, University Medical Center Hamburg-Eppendorf, Martinistrasse 52, 20246 Hamburg, Germany. E-mail: s.kluge{at}uke.de.
Fisher and Paykel (Auckland, New Zealand) provided the devices used for HFNC in this study. The authors have disclosed no conflicts of interest.
See the Related Editorial on Page 1273
- Copyright © 2016 by Daedalus Enterprises