Abstract
BACKGROUND: Volume-targeted ventilation is increasingly used in low birthweight infants because of the potential for reducing volutrauma and avoiding hypocapnea. However, it is not known what level of air leak is acceptable during neonatal volume-targeted ventilation when leak compensation is activated concurrently.
METHODS: Four ICU ventilators (Servo-i, PB980, V500, and Avea) were compared in available invasive volume-targeted ventilation modes (pressure control continuous spontaneous ventilation [PC-CSV] and pressure control continuous mandatory ventilation [PC-CMV]). The Servo-i and PB980 were tested with (+) and without (−) their proximal flow sensor. The V500 and Avea were tested with their proximal flow sensor as indicated by their manufacturers. An ASL 5000 lung model was used to simulate 4 neonatal scenarios (body weight 0.5, 1, 2, and 4 kg). The ASL 5000 was ventilated via an endotracheal tube with 3 different leaks. Two minutes of data were collected after each change in leak level, and the asynchrony index was calculated. Tidal volume (VT) before and after the change in leak was assessed.
RESULTS: The differences in delivered VT between before and after the change in leak were within ±5% in all scenarios with the PB980 (−/+) and V500. With the Servo-i (−/+), baseline VT was ≥10% greater than set VT during PC-CSV, and delivered VT markedly changed with leak. The Avea demonstrated persistent high VT in all leak scenarios. Across all ventilators, the median asynchrony index was 1% (interquartile range 0–27%) in PC-CSV and 1.8% (0–45%) in PC-CMV. The median asynchrony index was significantly higher in the Servo-i (−/+) than in the PB980 (−/+) and V500 in 1 and 2 kg scenarios during PC-CSV and PC-CMV.
CONCLUSIONS: The PB980 and V500 were the only ventilators to acclimate to all leak scenarios and achieve targeted VT. Further clinical investigation is needed to validate the use of leak compensation during neonatal volume-targeted ventilation.
- neonates
- mechanical ventilation
- acute care ventilator
- leak compensation
- volume-targeted ventilation
- patient-ventilator interaction
Introduction
Traditionally, time-cycled, pressure-limited ventilation is used in neonatal ventilation due to the decelerating gas flow pattern and the benefit of directly controlling peak inspiratory pressure.1 One disadvantage of pressure-limited ventilation is the variable tidal volumes that result from changes in lung compliance. Volume-targeted ventilation is a modification of pressure-limited ventilation that allows targeting of delivered tidal volume (VT) as a result of the development of sensitive and accurate flow sensors.2–4 Volume-targeted ventilation is increasingly used in extremely low birthweight infants because of the potential for reducing volutrauma and avoiding hypocapnea.1,4–6 A Cochrane review concluded that volume-targeted ventilation resulted in significant reductions in the duration of ventilation, rate of pneumothorax, and severe intraventricular hemorrhage compared with infants ventilated using pressure-limited ventilation.5,6
A major problem of mechanical ventilation in neonates is air leak because of the use of uncuffed endotracheal tubes (ETT).7 One of the major causes of triggering and cycling asynchrony is the presence of air leaks, which interferes with the ventilator's response to patients' spontaneous breathing efforts.8,9 Work of breathing and the duration of mechanical ventilation are directly affected by asynchronous patient-ventilator interactions.10,11 Also, an air leak potentially affects the operation of volume-targeted ventilation. Because expiratory VT measurement underestimates the actual delivered VT with large leaks, volume-targeted ventilation is generally recommended with ETT leaks up to 50%.2–4 Recently, manufacturers have implemented leak compensation algorithms on the latest acute care ventilators to compensate for leaks, and these algorithms can be activated during volume-targeted ventilation modes of some ventilators. To date, it is unclear how accurately VT is delivered during neonatal volume-targeted ventilation when leak compensation is activated concurrently.
Previously, we assessed leak compensation and showed improved triggering and cycling synchronization in adult,12 pediatric,13 and neonatal14 settings; however, these studies were tested in conventional patient-triggered ventilation but not in volume-targeted ventilation. The aim of this bench study was to evaluate the capability of leak compensation of all-age ICU ventilators during neonatal volume-targeted ventilation in terms of (1) difference in delivered VT before and after leak was introduced and (2) prevention of asynchronous events. All-age ICU ventilators were the only ventilators evaluated in this study because our previous data indicated that all-age ventilators performed as well as neonatal ventilators.15
QUICK LOOK
Current knowledge
Volume-targeted ventilation allows effective control of delivered tidal volume compared with conventional pressure-limited ventilation. Leaks affect delivered tidal volume accuracy and can cause trigger and cycling asynchrony.
What this paper contributes to our knowledge
Leak compensation performance demonstrated huge variations among all-age ICU ventilators during neonatal volume-targeted ventilation in terms of both accuracy of delivered tidal volume and prevention of patient-ventilator asynchronous events.
Methods
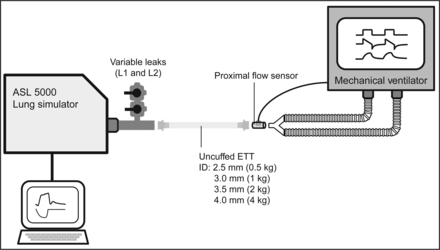
Four all-age ICU ventilators (Servo-i [Maquet, Wayne, New Jersey], PB980 [Covidien, Mansfield, Massachusetts], Evita Infinity V500 [Dräger, Telford, Pennsylvania], and Avea [CareFusion, San Diego, California]) (Table 1) were compared using an ASL 5000 lung simulator (version 3.5, IngMar Medical, Pittsburgh, Pennsylvania) with increasing and decreasing system leaks. Three-way stopcocks (Discofix, B. Braun Medical, Bethlehem, Pennsylvania) placed between an ETT and the lung simulator were used to create different leak levels (Fig. 1). The connection between the lung simulator and each ventilator was via the manufacturer's standard neonatal circuit or a standard neonatal circuit (Neonatal Breathing Circuit, Hudson RCI-Teleflex, Morrisville, North Carolina). Ventilators were studied with a dry circuit.
Specifications for Neonatal Use of Mechanical Ventilators Tested
Experimental setup. L1 = leak 1; L2 = leak 2; ETT = endotracheal tube; ID = inner diameter.
Lung Model and Study Setup
Four neonatal ventilation scenarios, with different lung sizes and mechanics estimated based on body weight (0.5, 1, 2, and 4 kg) were simulated. Table 2 summarizes the settings of the ASL 5000 in each evaluated scenario. The following variables are listed for each scenario: inspiratory time with the time percentages of a single breath cycle for the pressure drop (inspiratory), pressure maintenance (hold), and relaxation (expiratory); the maximum inspiratory pressure drop and the airway occlusion pressure; and the breathing frequency, resistance, and compliance. Previous neonatal bench studies16–19 as well as clinical studies of lung function tests in preterm infants with severe respiratory failure20–26 were used to select compliance and resistance values for each model (compliance, 0.4–1.3 mL/cm H2O/kg; resistance, 61–355 cm H2O/L/s). The pressure drop across each endotracheal tube as measured by a ventilator tester (PTS 2000, Mallinckrodt, Dublin, Ireland) and average peak flow achieved during the study were used to calculate the resistance across each endotracheal tube. Inspiratory efforts in children vary between waking and sleeping,27–29 especially in critically ill premature infants.16,30 Therefore, to account for this variability, we chose a smaller maximum inspiratory pressure drop than normally reported values in neonates.31
Lung Model Setup Used With ASL 5000
Uncuffed endotracheal tubes (2.5-mm internal diameter for 0.5 kg, 3.0-mm internal diameter for 1 kg, 3.5-mm internal diameter for 2 kg, and 4.0-mm internal diameter for 4 kg) were used to directly affix the lung model to each ventilator. A baseline leak of 0 L/min was established by vertically cutting the tip of each endotracheal tube and tightly connecting it to a common airway (Fig. 1). Two intentional leak levels (leak 1 and leak 2) were set to 0.1 and 0.2 L/min (50 and 95% of minute ventilation) for 0.5 kg; 0.2 and 0.4 L/min (56 and 111%) for 1 kg; 0.3 and 0.6 L/min (50 and 100%) for 2 kg; and 0.4 and 0.8 L/min (42 and 83%) for 4 kg. Each leak volume was calibrated with the PTS 2000 connected between the ASL 5000 and the leak system at a constant negative airway pressure of 5 cm H2O maintained by the ASL 5000. Since our aim was to evaluate the maximum ability of the leak compensation algorithms of each ventilator, leak settings higher than clinically reported32,33 but within the claims of the manufacturers (Table 1) were selected. All 3 combinations of increasing leak change (baseline leak→leak 1, baseline leak→leak 2, and leak 1→leak 2) and all 3 combinations of decreasing leak change (leak 2→leak 1, leak 2→baseline leak, and leak 1→baseline leak) were evaluated. All combinations of increasing and decreasing leaks were evaluated because in our previous studies12,13 we observed that the direction of the leak change and the magnitude of the leak change affected the ability of a ventilator to compensate for leaks. Before the study, the ASL 5000 was checked repeatedly for accuracy of tidal volume measurement by insufflating air from syringes of various volumes.
Ventilator Settings
All ventilators were tested in available volume-targeted pressure control continuous spontaneous ventilation (PC-CSV) and volume-targeted pressure control continuous mandatory ventilation (PC-CMV) modes. The Servo-i and PB980 were tested with (+) and without (−) their proximal flow sensor, since its use is optional. The V500 and Avea were always tested with the proximal flow sensor in accordance with the manufacturer's instructions. In both PC-CSV and PC-CMV, tidal volume (set VT) was set at 6 mL/kg, and PEEP was set at 5 cm H2O. To ensure that our methods could be easily reproduced, flow trigger sensitivity was set to be as sensitive as possible while avoiding auto-triggering at baseline leak, and inspiratory rise time was set to the fastest setting in all ventilators tested. Leak compensation was always activated. The upper pressure limit was set at the maximum value possible. During PC-CSV, the termination criteria were set at a level to obtain total inspiratory time of the ventilator (Tivent; time from the start of effort to the moment the ventilator cycled from inspiration to exhalation) equal to ±20% of the inspiratory time of the simulator (Tisim; increase (%) plus hold (%) in the effort model). During PC-CMV, the ventilator frequency was set at 35, 30, 25, and 20 breaths/min, and inspiratory time was set at 250, 300, 350, and 400 ms/min for the 0.5, 1, 2, and 4 kg models, respectively.
Apnea backup ventilation was activated with the apnea interval 20 s. Since criteria of automatic return from backup ventilation differed between ventilators, if backup ventilation was activated, we immediately reset the ventilator and waited for spontaneous recovery.
Data Collection and Evaluation
Up to a 2-min waiting period was allowed for the simulator to consistently trigger the ventilator during baseline leak before data gathering. If triggering was established, all combinations of increasing and decreasing leaks were sequentially added to the system (baseline leak→leak 1→leak 2→leak 1→baseline leak→leak 2→baseline leak). After each change in leak level, we collected 2 min of data from the time of change in leak level. Delivered VT (mL), peak flow (L/min), peak pressure (cm H2O) and PEEP (cm H2O) were recorded by the ASL 5000 for 2 min after each change in leak. The delivered VT before and after the change in leak (increase, baseline leak→leak 1; decrease, leak 1→baseline leak) was assessed as follows: (1) VT before the change (%): average VT (% of set VT) of 5 consecutive, normally triggered breaths just before each change in leak; (2) VT after the change (%): average VT (% of set VT) of last 5 consecutive, normally triggered breaths 2 min after each change in leak; (3) ΔVT (%): average VT difference (% of set VT) between before and after the change in leak.
The global asynchrony index7,34–36 was calculated as follows: asynchrony index = [(auto-triggering + double-triggering + ineffective efforts + premature cycling + delayed cycling during 2 min)/(total simulated breaths +auto-triggering)] × 100.
Synchronization was defined as triggering without auto-triggering, double-triggering, ineffective efforts, premature cycling, and delayed cycling. Asynchrony events were detected by visual inspection of flow and airway pressure recordings. Asynchrony events were defined according to previous studies7,34–36:
Auto-triggering: a cycle delivered by the ventilator in the absence of a signal generated by the lung simulator;
Double-triggering: 2 ventilator-delivered cycles separated by a very short expiratory time occurring within a single inspiratory effort of the lung simulator;
Ineffective efforts: inspiratory effort of the lung simulator not followed by a ventilator-delivered cycle;
Delayed cycling: a cycle normally triggered by the ventilator but with Tivent greater than twice the Tisim;
Premature cycling: a cycle normally triggered by the ventilator but with Tivent less than one-half the Tisim.
When backup ventilation operated, simulated breaths during backup ventilation were counted as ineffective efforts.
Statistical Analysis
Data were collected by the lung simulator's software (ASL 3.5), and each breath was manually analyzed to count asynchronous events by a single non-blinded observer. Results are expressed as mean ± SD or medians with interquartile ranges depending on the parametric or non-parametric nature of the data distribution. A one-way analysis of variance with the Tukey honest significant difference post hoc test was used for parametric data, and the Kruskal-Wallis one-way analysis by ranks and the Dunn test for multiple comparisons were used for non-parametric data. Statistical analysis was conducted using R Statistical Software (R Foundation for Statistical Computing, Vienna, Austria). A value of P < .05 was considered statistically significant. We report only differences that were both statistically significant (P < .05) and clinically important (>10%).
Results
Delivered VT
Tables 3 and 4 show delivered VT before and after the change in leak and ΔVT during PC-CSV and PC-CMV. The Servo-i and Avea experienced significantly frequent asynchronous events at leak 2; thus, only VT data at baseline leak and leak 1 are shown in Tables 3 and 4. Some VT data are not shown because 5 consecutive normally triggered breaths could not be obtained due to frequent asynchronous events with the specific leak.
Delivered Tidal Volume Before and After the Change in Leak During Volume-Targeted Pressure Control Continuous Spontaneous Ventilation
Delivered Tidal Volume Before and After the Change in Leak During Volume-Targeted Pressure Control Continuous Mandatory Ventilation
In PC-CSV, mean delivered VT at the baseline leak (evaluating VT at baseline leak before changing to leak 1) was ≥10% greater than set VT in all scenarios with the Servo-i (−) and Servo-i (+) (Table 3). When leak increased and decreased, ΔVT was statistically significant (P < .01) and clinically important (absolute change >10%) in the 2 kg scenarios with the Servo-i (−) and Servo-i (+) but not in 4 kg. ΔVT was consistent with the PB980 and V500 regardless of body weight, the use of a proximal flow sensor, and the direction of change in leak.
In PC-CMV, mean delivered VT at the baseline leak was generally within ±10% of set VT in all ventilators (Table 4). However, delivered VT was 18% greater than set VT in the 4 kg scenario with the PB980 (−) and PB980 (+) and 15% smaller than set VT in the 4 kg scenario with the V500. ΔVT was maintained within ±5% in all scenarios with the PB980 (−), PB980 (+), and V500 when leak increased and decreased. In all scenarios with the Avea, both increase and decrease in leak caused statistically significant (P < .01) and clinically important (absolute change >10%) changes in VT. ΔVT was consistent regardless of body weight, the use of a proximal flow sensor, and the direction of change in leak with the PB980 (−), PB980 (+), V500, and Avea. The characteristics of acute change in delivered VT when leak increased and decreased are demonstrated in the supplementary materials at http://www.rcjournal.com.
Performance of Leak Compensation on Synchrony
All ventilators were triggered by inspiratory flow from the simulator in all tested conditions under the baseline leak. Across all ventilators, median asynchrony index was 1% (interquartile range 0–27%) in PC-CSV (Fig. 2A) and 1.8% (0–45%) in PC-CMV (Fig. 2B). The asynchrony index varied widely, especially in scenarios where ventilators did not compensate for leaks at a higher level. There was no significant difference in asynchrony index between PC-CSV and PC-CMV in all tested ventilators. The asynchrony index tended to decrease but not significantly as body weight increased across all ventilators during PC-CSV (P = .36) and PC-CMV (P = .47). In PC-CSV, median asynchrony index was significantly higher in the Servo-i (−) and Servo-i (+) than the PB980 (−), PB980 (+), and V500 in 0.5, 1, and 2 kg scenarios except for the 0.5 kg scenario versus the V500 (P < .05 for all comparisons, Fig. 2A). In PC-CMV, median asynchrony index was significantly higher in the Servo-i (−) and Servo-i (+) than in the PB980 (−), PB980 (+), and V500 in the 1, 2, and 4 kg scenarios and also higher than the Avea in the 4 kg scenario (P < .05 for all comparisons, Fig. 2B). With the Servo-i and PB980, there was no relationship between asynchrony index and the use of a proximal flow sensor in any scenarios during both modes.
Asynchrony index (%) during volume-targeted ventilation relative to body weight. A: Volume-targeted pressure control continuous spontaneous ventilation. B: Volume-targeted pressure control continuous mandatory ventilation. Each box represents the interquartile range, with the median value shown as a horizontal line in the box. Error bars represent the maximum and minimum values. Data across all leak levels were compared between ventilators at each body weight. The Servo-i and PB980 were used with and without the proximal flow sensor. * = P < .05 vs PB980 without sensor, † = P < .05 vs PB980 with sensor, ‡ = P < .05 vs V500; all were clinically significant (>10%).
Across all ventilators, asynchrony index increased with leak in PC-CSV (P < .05, Fig. 3A) and tended to increase but not significantly with leak in PC-CMV (P = .064, Fig. 3B). In PC-CSV, asynchrony index was significantly higher with the Servo-i (−) and Servo-i (+) than the other ventilators at the leak 1 and leak 2 levels (Fig. 3A). In PC-CMV, the asynchrony index was significantly higher with the Servo-i (−), Servo-i (+), and Avea than the other ventilators at the leak 1 and leak 2 levels except for the Avea at leak 1 (Fig. 3B).
Asynchrony index (%) during volume-targeted ventilation relative to leak level. A: Volume-targeted pressure control continuous spontaneous ventilation. B: Volume-targeted pressure control continuous mandatory ventilation. Each box represents the interquartile range, with the median value shown as a horizontal line in the box. Error bars represent the maximum and minimum values. Data across all body weights were compared between ventilators at each leak level. The Servo-i and PB980 were used with and without the proximal flow sensor. * = P < .05 vs PB980, † = P < .05 vs V500, ‡ = P < .05 vs PB840, § = P < .05 vs Avea; all were clinically significant (>10%).
Cause of Asynchrony
Delayed cycling was seen only during PC-CMV, and there was no premature cycling in any tested conditions. Auto-triggering was the most common cause of asynchrony during PC-CSV, and delayed cycling was the most common cause of asynchrony during PC-CMV (Fig. 4, A and B). In PC-CSV, the incidence of both auto-triggering and double-triggering was significantly higher with the Servo-i (−) (auto-triggering, 26.3%; double-triggering, 12.4%) and Servo-i (+) (auto-triggering, 26.8%; double-triggering, 11.6%) when compared with the V500 for auto-triggering (0.3%, P < .001 for all comparisons) and PB980 (−) for double-triggering (0%, P < .005 for all comparisons) (Fig. 4A). In PC-CMV, the incidence of auto-triggering was significantly higher with the Servo-i (−) (23.1%), Servo-i (+) (22.1%), and Avea (21.1%) when compared with the V500, which had the lowest rates of auto-triggering (0.3%, P < .05 for all comparisons) (Fig. 4B). There was no significant difference in the rate of delayed cycling.
The cause of asynchrony during volume-targeted ventilation and total asynchrony index. A: Volume-targeted pressure control continuous spontaneous ventilation. B: Volume-targeted pressure control continuous mandatory ventilation. The bars show total asynchrony index (%). Each asynchrony type across all body weights and leak levels was compared. The Servo-i and PB980 were used with and without the proximal flow sensor. A: * P = < .001 vs V500 for auto-triggering, † = P < .001 vs PB980 without sensor for double-triggering; clinically significant (>10%). B: * = P < .05 vs V500 for auto-triggering, ‡ = P < .001 vs PB980 without sensor for double-triggering; clinically significant (>10%). Each asynchrony was compared with the lowest percentage for that type of asynchrony.
Peak Flow and Peak Pressure During Variable Leak
Across all body weights, peak flow and peak pressure significantly increased as the leak increased only with the Avea, and PEEP did not demonstrate a relationship with leak level in any of the tested ventilators. Data are shown in the supplementary materials.
Discussion
The main findings of this study are as follows: (1) the PB980, V500, and Avea showed acceptable baseline VT within ±10% of set VT in almost all tested conditions; (2) the overall effect of leak on delivered VT accuracy was minimal with the PB980 and V500; (3) with the Servo-i, baseline VT was ≥10% greater than set values during PC-CSV, and markedly changed with leak; (4) with the Avea, leak significantly affected delivered VT accuracy, resulting in persistent volume overshooting; (5) the PB980 and V500 showed significantly lower asynchrony index than the Servo-i during volume-targeted PC-CSV and PC-CMV; (6) auto-triggering and delayed cycling were the most common causes of asynchrony during volume-targeted PC-CSV and PC-CMV, respectively; and (7) the use of a proximal flow sensor did not affect ΔVT, asynchrony index, or type of asynchrony.
The Accuracy of Delivered VT
Previously, Jaecklin et al37 evaluated in their neonatal bench study whether volume-targeted ventilation can deliver a constant VT following sudden changes in compliance and resistance of the respiratory system and system leak. In this study, they observed volume overshooting after a rapid increase in compliance or decrease in resistance. However, changes in delivered VT after a change in leak were minor in all tested neonatal ventilators. The reason why this result is inconsistent with our findings can be explained by some differences in study conditions. First, they switched off automatic leak compensation because of inconsistent breath-to-breath variation in delivered VT and peak inspiratory pressure, which we did not observe. Second, they used smaller leak changes of 20–30% than those of our study (about 50–100%). High leak level was enough for the Servo-i and Avea to demonstrate frequent asynchronous events even if leak compensation was activated. A sudden drop of VT caused by ineffective efforts or an increase in VT caused by double-triggering could not be handled by volume-targeted ventilation because its algorithm limits the pressure increment from one breath to the next to a maximum of 3 cm H2O to avoid overcorrection leading to excessive VT and oscillations of the system.2,3
We observed significant volume overshooting during baseline (no leak) in all Servo-i runs during PC-CSV regardless of the use of a proximal flow sensor. Contrary to this, Jaecklin et al37 during PC-CMV observed VT smaller than set values during baseline (no leak) with the Servo-i. Differences in the airway pressure waveforms at the same volume-targeted ventilation settings are supposed to be the reason for variable delivered VT.38 Although it is unclear whether concurrent use of leak compensation or a difference in the VT monitoring caused this discrepancy, further investigations and improvements of the algorithms are needed, since prevention of hyperventilation is one of the chief goals of volume-targeted ventilation.1,4–6 We observed an improvement in accuracy of delivered VT by adding leak, especially when volume-overshooting at the baseline leak and inadequate leak compensation coexisted (eg, 2 kg scenario with the Servo-i [+] during PC-CSV). It may be possible that focusing only on delivered VT during volume-targeted ventilation makes the ability of leak compensation in each ventilator difficult to predict.
On the other hand, with the PB980 and V500, ΔVT remained within ±10% in all tested conditions, not only when leak volume was 50% but also at 100%. The ventilators that we evaluated had a greater capacity to compensate for leaks, and volume-targeted ventilation continued to operate appropriately.
The Incidence of Asynchrony
To our knowledge, this is the first study to assess leak compensation algorithms during neonatal volume-targeted ventilation in terms of prevention of asynchronous events. Vignaux et al clinically investigated asynchronous events in infants and children and reported asynchrony index of 24% in invasive PC-CSV34 and 40% in noninvasive PC-CSV35 even after adjustment of the termination criteria. In our previous neonatal bench study,14 we observed an asynchrony index of 29% during conventional PC-CSV and PC-CMV and observed that the asynchrony index was significantly lower with the PB980 (1%) and V500 (3%) than with the Servo-i (50%) and Avea (56%). Although we studied different modes, we observed similar frequency of asynchrony (40% asynchrony index with the Servo-i). In view of the fact that an asynchrony index >10% has been considered severe in previous studies,7,34,35 the appropriateness of neonatal patient-triggered modes including volume-targeted ventilation on some ICU ventilators must be questioned even if leak compensation is used concurrently.
Cause of Asynchrony
Generally, in PC-CSV, a large ETT leak may not allow the patient to flow-cycle to exhalation.39 However, delayed cycling was not seen during PC-CSV regardless of the presence of leak once we adjusted the termination criteria at baseline to ensure that the ventilator and simulator ended inspiration at the same time. This suggests that variable flow of all ventilators could match up with the leaks enough to achieve flow-cycling. On the other hand, we used fixed inspiratory times for the ventilator equal to that of the simulator in PC-CMV; thus, the timing of cycle-off criteria being met resulted in delayed cycling in the smallest infant scenarios, which have been shown to have a prolonged trigger delay time.28,40 This suggests that we may need to pay extra attention to the inspiratory time setting to avoid cycling asynchrony in premature time-cycled ventilation and that volume-targeted PC-CSV may be a better choice than volume-targeted PC-CMV.
Leak has been shown to be a major factor leading to auto-triggering,8,9,28 as we observed during PC-CSV. However, the frequency of auto-triggering did not increase in proportion to the leak level in PC-CSV. This is consistent with a previous clinical study, which demonstrated that auto-triggering occurs regardless of the leak volume, once leak flow reaches the trigger threshold.40 In PC-CMV, we observed double-triggering more often than auto-triggering at high leak level. Auto-triggered breaths were cycled in the middle of the lung model's inspiration, which resulted in a second triggering. With high leak level, auto-triggered breaths started even before the start of the patient's inspiration, which may result in more frequent double-triggering.
Effects of Proximal Flow Sensor
We did not observe a significant effect of the proximal flow sensor on ΔVT with the PB980. If an internal flow sensor can measure leak volume as accurately as a proximal flow sensor at the same leak level, the same compensation will be applied, and ΔVT may not change. It was difficult to evaluate the effect of the proximal flow sensor on ΔVT with the Servo-i due to high asynchrony index during the leaks tested. Rapidly developing leaks may require a few breaths before full compensation; thus, the potential of auto-triggering before compensation is maximized. Patient-ventilator synchrony significantly changes delivered VT, which may be a reason why a leak level <50% is suggested during volume-targeted ventilation.2–4
Limitations
There are several limitations to this study. First, this study was not conducted on patients, which raises the question of whether the findings are clinically important. However, it is impossible to control the level of leak in neonatal ventilation, and bench studies using the ASL 5000 ensure the same experimental condition for each ventilator. Second, parameters of our lung models potentially may not fit all patients with these body weights. Especially in children, morbid lung mechanics of patients with the same body weight generally differ and depend on their conditions.16,28–31 Third, a single-compartment model in the ASL 5000 was used. The ASL 5000 allows simulated non-linear resistance only in the dual-compartment model; thus, our lung models had linear resistance. This may affect the response of the tested ventilators in infants, since infants with lung disease frequently have non-linear resistance.41 Fourth, we tested only a limited higher range of leaks and ventilator settings. However, we intended to evaluate the maximum capabilities of leak compensation during volume-targeted ventilation for each ventilator by selecting leak volumes that were large but also likely to be encountered in clinical settings. Fifth, we do not know whether there are any inappropriate interactions between the volume-targeted ventilation algorithm and the leak compensation algorithm. Further studies to compare volume-targeted ventilation with and without leak compensation may reveal how much improvement can be obtained in each ventilator when leak compensation is activated. Finally, we did not record VT displayed by the ventilators; thus, it is impossible to determine how much underestimation of VT is commonly seen by critical caregivers2–4 during volume-targeted ventilation under the conditions we simulated. We focused on delivered VT because it is the determinant of patients' outcome related to volume-targeted ventilation.1,4–6
Conclusions
We observed a huge variation in the capability of leak compensation among all tested ventilators during neonatal volume-targeted ventilation in terms of both prevention of asynchronous events and delivering preset VT. Clinicians should be aware of these differences among ICU ventilators when they use these ventilators for neonatal volume-targeted ventilation. The PB980 and V500 were the only ventilators to acclimate to all leak scenarios. Clinical investigation is needed to validate our findings during the combination of leak compensation and neonatal volume-targeted ventilation.
Footnotes
- Correspondence: Robert M Kacmarek PhD RRT FAARC, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Harvard Medical School, 55 Fruit Street, Boston, MA 02114. E-mail: rkacmarek{at}mgh.harvard.edu.
Supplementary material related to this paper is available at http://www.rcjournal.com.
This study was funded in part by a research grant from Covidien. Dr Kacmarek has disclosed relationships with Covidien, Venner Medical, and Orange Medical. The other authors have disclosed no conflicts of interest.
See the Related Editorial on Page 135
- Copyright © 2017 by Daedalus Enterprises