Abstract
BACKGROUND: This study aims to determine the relationship between tobacco use, inhalation injury, and ARDS in burn-injured adults.
METHODS: This study was an observational cohort of 2,485 primary burn admissions to a referral burn center between January 1, 2008 and March 15, 2015. Subjects were evaluated by methods used to account for mediation and traditional approaches (multivariable logistic regression and propensity score analysis). Mediation analysis examined both the (1) indirect effect of tobacco use via inhalation injury as the mediator on ARDS development and (2) the direct effect of tobacco use alone on ARDS development.
RESULTS: ARDS development occurred in 6.8% (n = 170) of the cohort. Inhalation injury occurred in 5.0% (n = 125) of the cohort, and ARDS developed in 48.8% (n = 83) of the subjects with inhalation injury. Tobacco use was 2-fold more common in subjects with ARDS. In the mediated model, the direct effect of tobacco use on ARDS, including interaction between tobacco use and inhalation injury, was not significant (odds ratio [OR] 1.63, 95% CI 0.91–2.92, P = .10). However, the indirect effect of tobacco use via inhalation injury as the mediator was significant (OR 1.61, 95% CI 1.25–2.07, P < .001), and the proportion of the total effect of tobacco use operating through the mediator was 55.6%. In the non-mediation models (multivariable logistic regression and propensity score analysis), which controlled for inhalation injury and other covariables, the OR for the association between tobacco use and ARDS was 1.84 (95% CI 1.22–2.81, P < .001) and 1.69 (95% CI 1.04–2.75, P = .03), respectively.
CONCLUSIONS: In mediation analysis, inhalation injury was the overwhelming predictor for ARDS development, whereas tobacco use has its strongest effect indirectly through inhalation injury. Patients with at least moderate inhalation injury are at greatest risk for ARDS development despite baseline risk factors like tobacco use.
Introduction
More than one million burn injuries occur yearly in the United States, and survivorship is worse when the injury is associated with inhalation injury.1 Prior epidemiologic studies have shown that nearly half of residential fires and fatalities are associated with cigarettes or combustible tobacco products, and house fires involving cigarettes account for approximately one third of fire fatalities in the United States.2–4 Internationally, smoking is the leading cause of residential or total fire death and is an independent risk factor for thermal injury.4,5 Inhalation injury accounts for approximately 5% of in-patient admissions for burns.6 Burn care has improved in recent years, and mortality in burn injuries with total body surface area between 30 and 39% was 16% in 2013.1 However, morbidity from organ dysfunction, such as ARDS, remains poorly characterized.
Patient cohorts have demonstrated that >25% of subjects with large burns or inhalation injuries who survive their injury develop ARDS.7,8 Cohort studies to examine important risk factors for ARDS in the setting of burn injury have been limited by small sample sizes or by including only military personnel.9,10 Risk prediction models that have incorporated tobacco use as a predictor in patients are mainly derived from medical cohorts with sepsis and pneumonia.7 Both active and passive tobacco use are independent risk factors for the development of ARDS in critically ill patients, including trauma patients.11–13 However, in burn injury, tobacco use commonly precedes the burn injury and can have both a direct effect on ARDS and an indirect effect on ARDS through the burn injury it preceded. Separating tobacco's direct effect on ARDS versus its indirect effects via other lung injury mediators (eg, inhalation injury) cannot be fully evaluated with traditional statistical methods that do not account for the temporality in the pathway between tobacco use, inhalation injury, and ARDS. Mediation analysis, a method adopted from social sciences, allows for evaluation of simultaneous regression pathways on an outcome. This method was employed to examine the impact of driving pressures within a lung-protective ventilation strategy that improved mortality in patients with ARDS.14
To further clarify the association between tobacco use, inhalation injury, and development of ARDS, we examined subjects from a high-volume regional referral burn center and burn research institute during a 7-y period. We hypothesized that burn injury itself was the overwhelming predictor of ARDS, and the risk contributed by tobacco use was not significant. We examined our hypothesis using traditional statistical approaches as well as mediation analysis to compare how tobacco use is associated with ARDS with and without inhalation injury as the mediator.
QUICK LOOK
Current knowledge
Prior studies examining tobacco use have identified it as a risk factor for ARDS in other critically ill patients. However, burn injury is less clear and requires analysis into the temporal relationship because smoking is an independent risk factor for inhalation injury and confers risk for ARDS through inhalation injury.
What this paper contributes to our knowledge
Inhalation injury was an overwhelming predictor for ARDS, with 56% of the increased risk due to inhalation injury as the mediator. This was observed despite interactions between tobacco use and inhalation injury as well as adjustment for total body surface area, mechanism of injury, and subject characteristics. Prospective clinical studies are needed to better examine the effect of inhalation injury over other risk factors so that clinicians may focus care to attenuate the strongest determinants for ARDS development.
Methods
We analyzed data from an observational cohort of consecutive burn-injured adults admitted between January 1, 2008 and March 15, 2015 to Loyola University Chicago Burn Center. Loyola University Chicago is a tertiary academic center and referral burn center treating nearly 700 patients annually. All patients admitted to the burn center were recorded in the institution's burn registry and maintained within the Burn and Shock Trauma Research Institute. All admissions, burn injury characteristics, and dates of burn injury were verified by 2 full-time clinical research nurses in the burn unit with >15 y of dedicated clinical and research experience. Additional clinical variables were extracted from the electronic medical record by linkage of the burn registry to the clinical research database.
Subject Selection and Main Outcome Measure
Patients ≥18 y of age and with a primary admission for burn were evaluated. Patients admitted for non-burn injuries and subsequent encounters for prior injuries were excluded. The primary exposure of interest, tobacco use disorder, was defined using administrative data for tobacco use from screening intake questionnaire data or claims data. Subject comorbidities, such as diabetes mellitus type I or II, hypertension, any respiratory disease, and congestive heart failure, were collected from administrative data. Percentage of total body surface area injured was verified after subject discharge by review of operative notes and attending documentation from the clinical research nurses. All subjects receiving invasive mechanical ventilation with suspected inhalation injury received a fiberoptic bronchoscopic examination to evaluate for severity of injury. During bronchoscopy, inhalation injury was graded based on abbreviated injury score.15
ARDS
The Berlin definition16 was applied via an automated electronic screening tool for identification of ARDS cases. The authors designed the tool in a structured query language-based integrative database from the clinical research database. Electronic medical records served as the main data source for the rules of development with linked mechanical ventilation data, arterial blood gas data, and chest imaging reports. The tool was adapted and updated from prior internal and external validation studies in trauma subjects using a rule-based keyword search approach.17 The authors have previously applied this tool in other trauma registries.18 In a simple random sample of 50% of the patients with inhalation injury from our burn registry, we demonstrated similar test characteristics with a sensitivity and specificity of 76.4 and 88% after review of the chest radiograph images by a pulmonary and critical care physician (MA). The approach adapted to the Berlin definition is shown in the Appendix (see the supplementary materials at http://www.rcjournal.com).
Covariables
Candidate variables were identified a priori as risk factors for ARDS,7,11,19–21 and their selection for possible inclusion in the adjusted model was made if they had a P value <.20 in univariable analysis. Candidate variables included age, sex, race, burn mechanism, tobacco use, diabetes, inhalation injury, total body surface area injured, and burn mechanism. Alcohol use was not evaluated for inclusion into the multivariable models due to a high proportion of missing data. Inhalation injury was categorized as a dichotomous variable (not indicated/abbreviated injury score = 0 vs abbreviated injury score ≥1). Total body surface area injury at 33% demonstrated the optimal cutoff for development of ARDS using receiver operating characteristic area under the curve with a sensitivity and specificity of 65.3 and 88.9%, respectively. Therefore, total body surface area injured was evaluated as a dichotomous variable at ≥33%. Burn mechanism was a dichotomous variable as flame versus non-flame because flame mechanism comprised 45.3% of the cohort. Due to the low rates of comorbidities, respiratory diseases were combined. The small sample size of any individual respiratory disease did not allow for examination of the role of different respiratory diseases on outcomes.
Analysis
In univariable analysis, continuous variables were evaluated as medians and interquartile ranges and analyzed using either Wilcoxon rank sum or Kruskal-Wallis nonparametric tests, whereas categorical variables were analyzed using a chi-square test or Fisher exact test, if appropriate. Three different models were used to examine the association between tobacco use and development of ARDS. In the first model, multivariable logistic regression was used to assess the association (odds ratio) between tobacco use and ARDS development with adjustment for age, sex, mechanism of burn, total body surface area injured, and inhalation injury. In the second model, 2:1 matched propensity score analysis was performed, with age, sex, mechanism of burn, total body surface area injured, and inhalation injury used to create the propensity. Using each matched set as a stratum, a conditional logistic regression model was applied to estimate the association of tobacco use and ARDS. In the third model, a pathway framework was used to decompose the total effect of tobacco use on ARDS and incorporate inhalation injury as the mediating effect.22 This included the effect of tobacco directly on ARDS (pure direct effect). A second pathway was the indirect effect of tobacco on ARDS through the mediator inhalation injury (pure indirect effect). These pathways included analysis that allowed for exposure-mediator (tobacco-inhalation injury) interaction, referred to as the total direct and total indirect effect, respectively (Fig. 1). The model also allowed for both exposure-outcome confounders (age, sex, total body surface area injured) and mediator-outcome confounders (burn mechanism, total body surface area injured). Direct and indirect effects on the odds ratio scale was estimated via this approach. We used 500 bootstrap samples and the percentile method to produce 95% CIs. The same mediation analysis was performed for the flame subgroup, which is the predominant mechanism of burn injury. Other important mediator-outcome confounders, such as sepsis, have been shown to occur frequently in critically ill burn patients, so sensitivity analysis was performed for unmeasured confounding. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, North Carolina). The institutional review board of Loyola University Chicago approved this study.
A mediation model with exposure-mediator interactions. The filled circle represents an interaction term consisting of the variables connected to it without arrowheads, in this case tobacco exposure and inhalation injury. β1γ1 = indirect effect; β2 = direct effect; β3 = exposure-mediator interaction; γ0 = intercept. Effects in the model are shown above.
Results
A total of 2,485 subjects arrived at the burn center with a primary diagnosis for burn injury. The cohort case rate for development of ARDS was 6.8% (n = 170). The majority of admissions for burn were total body surface area injury < 10% (n = 2,008, 80.8%), but 44.1% (n = 75) of the ARDS cases occurred in this group, of which 54.7% (n = 42) had inhalation injury. The median (interquartile range) time to ARDS development was 1.7 (0.4–3.6) d. Inhalation injury with or without burn injuries occurred in 5.0% (n = 125) of the cohort, and ARDS development occurred in 48.8% (n = 83) of these subjects. Over 90% (n = 113) of the inhalation injury subjects with ARDS had at least moderate inhalation injury (abbreviated injury score ≥2). Few subjects in the cohort had prior respiratory or cardiac disease (Table 1). Subjects with ARDS were older and had more comorbidities than their counterparts without ARDS. Burn injury characteristics were more severe in the ARDS group. These subjects habitually consumed more alcohol and had nearly 2-fold more tobacco use than those without ARDS (Table 1).
Subject Characteristics and Outcomes by ARDS Group
Outcomes Between ARDS and Non-ARDS Patients
The mean 28-d ICU-free days was lower in ARDS subjects than their non-ARDS counterparts (4.4 vs 20.5 d, P < .001), and as expected, the in-hospital case fatality rate in subjects with ARDS was much higher (25.3% vs 2.2%, P < .001). In burn subjects with ARDS, the median PaO2/FIO2 was similar between tobacco and non-tobacco users (189.8 vs 186.2, P = .65). Furthermore, the 28-d ICU-free days and in-hospital death rate were similar between tobacco and non-tobacco users with ARDS (P = .44 and .25, respectively).
Association Between Tobacco Use, Inhalational Injury, and ARDS
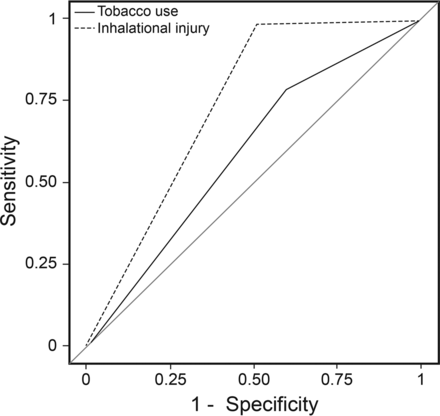
In multivariable logistic regression analysis adjusted for age, sex, burn mechanism, total body surface area injured, and inhalation injury score (Fig. 2), the OR for the association between tobacco exposure and ARDS development was 1.84 (95% CI 1.22–2.81, P < .001). Inhalation injury had the highest odds for ARDS development among the risk factors (OR 37.08, 95% CI 22.97–59.86, P < .001), followed by total body surface area injured ≥33% (OR 12.92, 95% CI 6.88–24.26, P < .001). An increasing inhalation injury score was associated with increasing risk for ARDS development (P < .001). Inhalation injury also provided better discrimination for the development of ARDS than tobacco use, with a receiver operating characteristic area under the curve of 0.74 (95% CI 0.70–0.77) versus 0.59 (95% CI 0.55–0.63), P < .001 (Fig. 3). In propensity score analysis (n = 1,130), the association between tobacco use and ARDS development remained significant, with an OR of 1.69 (95% CI 1.04–2.75, P = .034) (see Fig. 2).
Multivariable logistic regression with tobacco use as the independent variable and ARDS as the dependent variable. Age, sex, mechanism of burn, percentage of total body surface area injured, and inhalation injury are covariables in the model. Shown is propensity score analysis with conditional logistic regression after 2:1 matching of the covariables age, sex, percentage of total body surface area injured, burn mechanism, and inhalation injury between non-tobacco users and tobacco users for the association with ARDS.
Receiver operating characteristic curves comparing inhalation injury and tobacco use for discriminating ARDS development. Areas under the curve are 0.59 for tobacco use and 0.74 for inhalational injury.
However, in the mediated model, the total direct effect of tobacco exposure on ARDS, including interaction between tobacco use and inhalation injury, was not significant (OR 1.63, 95% CI 0.91–2.92, P = .10). The pure indirect effect of tobacco exposure via inhalation injury as the mediator between tobacco use and ARDS development was significant (OR 1.61, 95% CI 1.25–2.07, P < .001) (Fig. 1). The proportion of ARDS development mediated by inhalation injury in the total effect was 55.6%. Comparison of all 3 models is shown in Figure 4.
Comparison of unmediated and mediated models for the relationship between tobacco exposure and ARDS development.
Analysis Stratified to Flame Injury Only
In sub-analysis involving only flame injuries (n = 1,125), tobacco use had an increased OR for ARDS development in multivariable logistic regression (OR 2.01, 95% CI 1.24–3.26, P < .001), and inhalation injury continued to have the greatest OR (20.17, 95% CI 11.51–35.33, P < .001) for development of ARDS. In mediation analysis, the total direct effect from tobacco use on ARDS development was significant (OR 2.83, 95% CI 1.08–7.39, P = .042), but the total indirect effect with inhalation injury as the mediator continued to have the strongest association (OR 1.74, 95% CI 1.27–2.40, P < .001), and the proportion of ARDS development mediated by inhalation injury in the total effect was 57.0%, similar to the analysis from the full cohort (Fig. 5). In sensitivity analysis, we assumed that sepsis occurred in 30% of ARDS cases versus 7% in non-ARDS cases, as reported in prior studies,23,24 and the case rate for ARDS in smokers versus non-smokers in our cohort was 40% versus 22%. The total direct effect remained insignificant, with an OR of 2.11 (95% CI 0.81–6.84).
A mediation model with exposure-mediator interactions for the analysis stratified to flame injury only (n = 1,125). The filled circle represents an interaction term consisting of the variables connected to it without arrowheads, in this case tobacco exposure and inhalation injury. β1γ1 = indirect effect; β2 = direct effect; β3 = exposure-mediator interaction; γ0 = intercept. Effects in the model are shown above.
Discussion
Our data using mediation analysis demonstrated most of the association between tobacco use and ARDS development to be mediated through inhalation injury with limited contribution from tobacco use itself. Commonly used statistical approaches like multivariable logistic regression and propensity score analysis are limited in their ability to account for intermediate outcomes (ie, inhalation injury) in determining associations between exposure (ie, tobacco use) and outcomes of interest (ie, ARDS). Results from these models suggest that tobacco use is an important risk factor for ARDS development in burn-injured adults. In contrast, mediation analysis allows for intermediate outcomes in analyzing the direct and indirect associations between tobacco use, inhalation injury, and ARDS and therefore more accurately approximates the risk from tobacco use before burn/inhalation injury.
This study cohort had few comorbidities and experienced minor burns that probably contributed to the low case rate for ARDS. Prior studies in trauma cohorts with inclusion of all encounters have demonstrated similar rates of ARDS development between 5 and 10%.7,23,24 Similar rates from the National In-Patient Sample and the National Burn Repository of in-patient admissions for burn with concomitant inhalation injury were noted around 5%.6 However, our study revealed that nearly half of the cohort suffering from inhalation injury developed ARDS, much higher than the 20% reported in previous smaller studies.9,25 A prospective multi-center observational study with ARDS cases reviewed by pulmonary and critical care physicians confirmed a similar rate to this study at 43%.26 Despite a lower case rate, our burn-ARDS phenotype was older, were characterized by increased comorbidities, and had more substance use than the non-ARDS group, similar to ARDS phenotypes described previously.23,27
The prevalence of smoking in the United States population was estimated at 16.8% in 2015; our cohort of burn subjects had higher rates than the general United States population, with tobacco use present in approximately 25%. Epidemiologic studies have confirmed the increased risk for burn injury from cigarette smoking, and our cohort had an increased OR for inhalation injury in the mediated model. Tobacco use also had an increased odds risk for ARDS development, which was previously described in a trauma cohort.11 In some cases, tobacco use may have contributed to the injury as part of the indirect pathway toward ARDS, whereas in other cases, tobacco use itself potentially modulated the innate immune response and inflammatory milieu of the lung independent of inhalation injury and consequently engendered ARDS. Experimental studies have demonstrated that active smoking leads to pathophysiological changes similar to those in ARDS, including increased lung epithelial permeability, endothelial injury, and dysregulated platelet function.28–31 However, multiple outcomes in our subjects with ARDS were not affected by tobacco use, including PaO2/FIO2 ratio, 28-d ICU-free days, and in-hospital fatality rate. Although multivariable logistic regression and propensity score analysis was remarkable for a positive association between tobacco use and ARDS development, mediation analysis indicated that the indirect effect of tobacco use was most important via inhalation injury. On the OR scale, 56% of the increased risk was due to inhalation injury as the mediator. This was observed despite interactions between tobacco use and inhalation injury as well as adjustment for total body surface area injured, mechanism of injury, and subject characteristics. Because tobacco use may both predispose individuals to injury and influence the severity of injury, traditional adjustment for it probably introduced an overadjustment bias in multivariable logistic regression and propensity score analysis.22,32–34 Mediation analysis more accurately reflects the important exposures for ARDS and indicated that the majority of the effect from smoking on ARDS development was via inhalation injury as the mediator.
The results did vary when the analysis was stratified by flame injury to remove other burn mechanisms that probably do not suffer inhalation injury (ie, electrical, scald, contact). In the flame injury group, which comprised the largest subgroup in the cohort, the direct effect that included the effect of tobacco use on ARDS allowing for tobacco use and inhalation injury interaction was significant. However, like the analysis in the full cohort, the relationship remained the strongest via the indirect effect, which demonstrated a highly significant P value. Furthermore, 57% of the total effect was mediated by inhalation injury, similar to the full cohort. Nevertheless, it is possible that tobacco use had a significant direct effect in the flame-only group because patients injured from combustible tobacco products typically occur in settings like a house fire and present with flame mechanisms and inhalation injury, making the injuries more difficult to parse out and the interaction stronger.
Inhalation injury was an overwhelming predictor for ARDS, as characterized by better sensitivity and specificity for ARDS development and a dose-dependent increase in the OR for ARDS development with higher grades of inhalation injury. Several mechanisms from experimental studies of inhalation injury in both humans and animals established a dysregulated innate immune response in the airway.35–37 Increased permeability of the microvasculature in the setting of a 20-fold increased bronchial blood flow, intra-airway coagulation and fibrin deposition, cellular debris, obstructive airway casts, large volume resuscitation, and fibrinocellular exudate all contribute to ventilation and perfusion mismatching with maldistribution of alveolar volumes causing both atelectasis and barotrauma.38–43 Qualified chest radiograph reports using key words for ARDS definition may not discern between cardiogenic and proteinaceous pulmonary edema. Subjects with ARDS had larger percentage of total body surface area injured and worse inhalational injuries, so they probably received larger volumes of crystalloid resuscitation, probably contributing to false positives for ARDS cases. Inter-observer variability in chest radiograph interpretation commonly occurs in characterizing cardiogenic versus non-cardiogenic pulmonary edema; however, it is expected that hydrostatic edema in the form of cardiac failure or fluid overload may coexist with ARDS, and the Berlin definition accepts respiratory failure not fully explained by cardiac failure or fluid overload for ARDS definition. Although the false positive rates were low, they probably contributed to variability in our results.
The strengths of this study were its large size, verified injury characteristics, and validated ARDS diagnoses that are more robust than another large burn study based on administrative data alone.25 Several limitations were present in this study including its retrospective and single center design. The use of administrative data places limitations on the quality of the data, with missing data and biases that could not be accounted for. Although the ARDS screening system was previously validated, prior studies indicated that inter-observer reliability is poor between radiologists for pulmonary edema versus ARDS,44 so misclassification bias may have occurred. Tobacco use was assessed by administrative data alone, whereas prior studies confirmed tobacco use with biomarkers, including cotinine.11,12 Chronic alcohol use is an important risk factor for ARDS,19 and co-substance use between tobacco and alcohol are common; however, we did not have comprehensive data on alcohol use to examine these effects. Data for ventilator settings were not collected and could not be assessed in the evaluation for ARDS development. The practice behaviors of burn critical care physicians probably varied between practitioners, and the use of lung-protective ventilation strategies was not standardized. In addition, our study was limited by lack of data for sepsis and other infectious complications that have been reported to occur in nearly a third of critically ill burn patients.45 However, sensitivity analysis was performed to address this limitation. Our results did not change with the inclusion of sepsis as an unmeasured confounder.
Conclusions
Tobacco use constitutes an important risk for burn injury and is a common problem in burn-injured patients. Although tobacco use increased the odds of ARDS, the large majority of the risk for ARDS derives from the burn injury alone and, in particular, inhalation injury. In a subgroup of burn subjects with flame mechanism, tobacco had a significant direct effect on the development of ARDS, but the majority of the effect was still mediated through inhalation injury, similar to the full cohort. Prospective clinical studies are needed to better examine the effect of inhalation injury over other risk factors so that clinicians may focus care to attenuate the strongest determinants for ARDS development.
Acknowledgments
We thank the clinical research nurse Peggie Conrad for managing the burn registry and appreciate the Loyola Clinical Research Database leadership of Ron Price and programming by Susan Zelisko.
Footnotes
- Correspondence: Majid Afshar MD MSc, Loyola University Chicago, Center for Translational Research and Education, Room 447, 2160 South First Avenue, Maywood, IL 60153. E-mail: majid.afshar{at}luhs.org.
Dr Afshar presented a version of this work at the American Thoracic Society International Conference, held May 15–20, 2015, in Denver, Colorado.
This research was supported in part by National Institute of Alcoholism and Alcohol Abuse grants K23AA024503 (to MA) and R21 AA023193 (to EJK) and National Institute of General Medical Sciences grant R01 GM115257 (to EJK). The authors have disclosed no conflicts of interest.
Supplementary material related to this paper is available at http://www.rcjournal.com.
- Copyright © 2017 by Daedalus Enterprises