Abstract
BACKGROUND: ARDS is characterized by decreased functional residual capacity (FRC), heterogeneous lung injury, and severe hypoxemia. Tidal ventilation is preferentially distributed to ventilated alveoli. Aerosolized prostaglandin I2 exploits this pathophysiology by inducing local vasodilation, thereby increasing ventilation-perfusion matching and reducing hypoxemia. Therefore, aerosolized prostaglandin I2 efficacy may depend upon FRC. Both PaO2/FIO2 and compliance of the respiratory system (CRS) are indirect signifiers of FRC and thus may partly determine the response to aerosolized prostaglandin I2.
METHODS: We reviewed the records of 208 ARDS subjects who received aerosolized prostaglandin I2 and had arterial blood gases done before and after the initiation of therapy, without other ventilator manipulations. Subjects were grouped according to baseline PaO2/FIO2 (lowest: < 60, intermediate: 60–90, highest: > 90 mm Hg) and CRS (< 20, 20–29, 30–39, and ≥ 40 mL/cm H2O) and by other factors, such as sepsis. Comparisons were analyzed by paired t tests, or Kruskal-Wallis and Dunn post-tests. Multivariate logistic regression modeling was done to determine which of 18 clinically relevant factors were most predictive for responding to aerosolized prostaglandin I2. α was set at .05.
RESULTS: Mean PaO2/FIO2 increased by 33 mm Hg (42%) upon initiation of prostaglandin I2, with a responder rate of 62%. PaO2/FIO2 increased significantly in all oxygenation groups. The highest baseline PaO2/FIO2 group had the greatest improvement and responder rate (51 ± 63 mm Hg, and 82%). In addition, those with sepsis had a smaller improvement in PaO2/FIO2 compared with those without sepsis (18 ± 35 vs 40 ± 55 mm Hg, P = .002). Both PaO2/FIO2 and responder rate increased as CRS improved, but between-group improvements were not as consistent. In the final model, the only factors that predicted a positive response to aerosolized prostaglandin I2 were baseline PaO2/FIO2 (odds ratio 1.10 [1.004–1.205], P = .042) and CRS (odds ratio 1.04 [1.01–1.08], P = .02).
CONCLUSIONS: Aerosolized prostaglandin I2 improves oxygenation in approximately 60% of ARDS cases. A favorable response was most strongly associated with baseline PaO2/FIO2 and CRS.
Introduction
ARDS is characterized by severe hypoxemia from altered permeability pulmonary edema leading to decreased functional residual capacity (FRC), which in turn causes hypoxemia from intrapulmonary shunting, and areas of low alveolar ventilation to perfusion.1 Pulmonary hypertension is also a common feature of ARDS resulting from pulmonary vascular endothelial injury as well as from the effects of hypoxemia, hypercapnia, and acidosis that, if sustained, leads to cor pulmonale and increased mortality risk.2
Because lung injury in ARDS is non-homogeneous3, portions of the lungs may remain functionally normal, so that tidal ventilation is preferentially distributed to these alveoli. Inhaled vasodilators, such as nitric oxide (NO)4 and aerosolized prostaglandin I2,5 exploit this pathophysiology by inducing local pulmonary vasodilation, thereby increasing alveolar ventilation/perfusion matching.6 These agents also reduce pulmonary arterial pressure in ARDS.7 In addition, aerosolized prostaglandin I2 possesses both anti-inflammatory properties8 and anticoagulant properties.9 In theory, these characteristics may lessen the impact of pulmonary vascular endothelial injury and abnormal pro-coagulation that are prominent features of ARDS.10
Because of its heterogeneous nature, the extent and distribution of lung injury in ARDS is relatively unique in individual cases. Therefore, the effectiveness of aerosolized prostaglandin I2 in ARDS may be determined in part by the magnitude of FRC loss. In ARDS, mean FRC is reduced to between 1.8 and 0.6 L (or approximately 75 to 25% of normal).11 Because FRC is essentially the alveolar volume and the primary determinant of oxygenation and compliance of the respiratory system (CRS),12 we hypothesized that the initial response to aerosolized prostaglandin I2 in ARDS would be greater in those with less impaired oxygenation. We used both PaO2/FIO2 and CRS as indirect correlates of FRC status.
In addition, we evaluated whether ARDS etiology (particularly sepsis) or classification as direct versus indirect injury mechanisms modifies the response to aerosolized prostaglandin I2. Inhaled NO and aerosolized prostaglandin I2 cause vasodilation through similar pathways.13 Prior studies reported that inhaled NO was less effective in sepsis-associated ARDS,14,15 due to both the blunting effects of endogenous NO overexpression during sepsis14 and sepsis-induced cardiac dysfunction.15 Yet others reported that aerosolized prostaglandin I2 improved oxygenation only in those with indirect or extrapulmonary etiologies for ARDS (78% of whom had sepsis).16 Therefore, we also investigated the response to aerosolized prostaglandin I2 in ARDS subjects with and without evidence of sepsis.
QUICK LOOK
Current knowledge
Aerosolized prostaglandin I2 has been used to improve oxygenation in subjects with ARDS for > 20 y. Most studies have been small and generally have consisted of case series and retrospective reports. Various dosing regimens have been used and have produced modest-to-moderate improvements in oxygenation.
What this paper contributes to our knowledge
The majority of subjects administered aerosolized prostaglandin I2 had an improvement in oxygenation. Both the magnitude of response and the response rate decrease as the degree of oxygenation dysfunction worsens. This suggests that the efficacy of aerosolized prostaglandin I2 is dependent upon the amount of aerated lung. The response to aerosolized prostaglandin I2 was weaker in those with sepsis and stronger in those with trauma-associated ARDS.
Methods
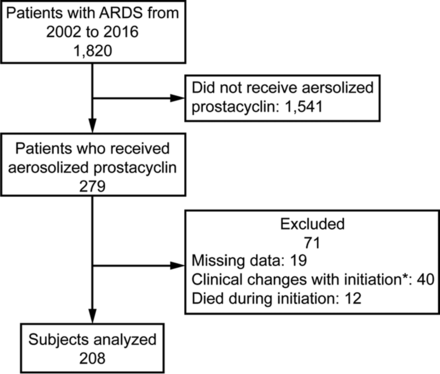
We utilized our hospital's ARDS quality assurance database to examine the effectiveness of aerosolized prostaglandin I2 in ARDS. This database registered all patients who met the American-European Consensus Conference definition of acute lung injury from June 2002 to April 2016.17 For the purposes of this study, all subjects were reclassified post hoc according to the current Berlin definition of ARDS.18 The database was reviewed to identify all patients who received aerosolized prostaglandin I2 during that time period. Approval to use our quality assurance data was granted by our institutional review board. Of the 1,820 patients in the database, 279 had received aerosolized prostaglandin I2. After excluding those who died during the therapy; had intervening increases in PEEP, recruitment maneuvers, or prone positioning; or had missing data/medical record, there were 208 subjects who had an arterial blood gas before and after the initiation of aerosolized prostaglandin I2 and thus were available for analysis (Fig. 1).
Flow chart. * = changes in PEEP, prone position, or recruitment maneuvers concurrent with aerosolized prostacyclin initiation.
Acute Physiology and Chronic Health Evaluation (APACHE II),19 Simplified Acute Physiology Score II (SAPS II),20 and lung injury scores21 were calculated on the day of ARDS onset. Basic demographic information as well as the primary etiology of ARDS and the presence of severe sepsis/septic shock also were collected. Lung injury score was again calculated just before initiating aerosolized prostaglandin I2. In addition, the medical records were reviewed to determine all potential mechanisms that might have contributed to the development of ARDS so that lung injury could be classified as being direct, indirect, or mixed. All subjects were managed with one of the ARDSNet ventilator protocols, which is a standard of care at our institution.22,23
The aerosol delivery system used was described previously.24 In brief, a 50-mL solution of prostacyclin (3.0 × 104 ng/mL) and a 500-mL normal saline solution were infused by a dual-channel volumetric infusion pump into a Mini-HEART (Westmed, Tucson, Arizona) jet nebulizer connected to the ventilator circuit with a T-adapter. We found this nebulizer to generate an aerosol with a mass median diameter of 3.12 ± 0.02 μm.24 In 2010, the delivery system was modified using an Aerogen (Mountain View, California.) vibrating mesh technology (mass median diameter of 3.1 μm)25 with a fixed concentration of prostacyclin infused through a single volumetric infusion pump. Therapy commenced at the highest recommended dose (50 ng/kg/min) and titrated downward. This approach was based on our early experiences with aerosolized prostacyclin indicating that subjects tend to respond quickly to the highest dose. Therefore, our strategy was based on clinical expediency.
Response to aerosolized prostaglandin I2 was assessed primarily by changes in PaO2/FIO2 and also by variables considered to indirectly reflect changes in physiologic dead space: namely the gradient between PaCO2 and end-tidal carbon dioxide tension and corrected minute ventilation (V̇E × PaCO2/40).26 Data were analyzed according to two categories used as signifiers of FRC impairment. The hypoxemia groups were determined a priori to ensure both reasonably approximate sample sizes and clinically informative cutoffs in baseline PaO2/FIO2 for severe ARDS and were classified as follows: lowest (< 60 mm Hg), intermediate (60–90 mm Hg), and highest (> 90 mm Hg). The compliance groups were based upon clinically informative cutoffs in CRS: < 20 mL/cm H2O, 20–29 mL/cm H2O, 30–39 mL/cm H2O, and ≥ 40 cm H2O. Marked improvement in oxygenation was defined pre hoc as a PaO2/FIO2 response of ≥ 10 mm Hg based on the observations of Walmrath et al.27
Statistical analysis was done using either Stata 9.0 (Stata Corp, College Station, Texas) or Instat (GraphPad Software, La Jolla, California). Data are reported as mean ± SD. Pre-post analysis of variables was done using paired t tests, whereas comparisons between 2 groups (eg, responders and non-responders) were done with unpaired t tests. For non-normally distributed data variables, comparisons were analyzed using either 2-sided Wilcoxon signed-rank tests (pre-post comparisons) or 2-sided Mann-Whitney tests (between-group comparisons). Multiple group comparisons were made using Kruskall-Wallis and Dunn's post-test. Categorical variables were assessed by chi-square tests. α was set at .05.
Backward, step-wise logistic regression modeling was used to determine which variables differentiated aerosolized prostaglandin I2 responders from non-responders. Variables included in the initial model were age, sex, ethnicity, APACHE II, SAPS II, lung injury score, ARDS etiology, injury category, Berlin definition category, duration of ARDS, presence of sepsis, mean arterial blood pressure, use of vasopressors (both dichotomous classification and number of agents), neuromuscular blockade, baseline PaO2/FIO2, CRS, VT, PEEP, and nebulizer type. The final model included all variables with a P ≤ .10.
Results
General Characteristics
The majority of our 208 study subjects presented with severe ARDS, as judged by lung injury score at the onset of ARDS and its deterioration at the time that aerosolized prostaglandin I2 was initiated (Table 1). The proportion of subjects classified as having severe ARDS according to the Berlin definition17 increased to over four fifths by the time aerosolized prostaglandin I2 was initiated. An equal proportion of subjects could be categorized as direct or indirect injury, whereas a quarter of all subjects had lung injury attributable to both mechanisms. No primary etiology of ARDS was disproportionately represented. Responders also had significantly lower APACHE II, SAPS II, and lung injury scores at ARDS onset (Table 2).
Demographics, Baseline Characteristics at ARDS Onset, and Outcome
Characteristics of Responders and non-Responders to Aerosolized Prostaglandin I2
Hemodynamics and the Response to Aerosolized Prostaglandin I2
Sixty-five percent of all subjects required vasopressors (Table 1). Of these, 36% required a single agent, 27% required a dual agent, 27% required a triple agent, 7% required 4 agents, and 2% required 5 agents. Although the proportion of subjects requiring vasopressors was not different between responders and non-responders, the later required significantly more vasopressor agents (Table 2).
With the exception of higher epinephrine and vasopressin dosages (which reflected 5 subjects who started therapy after aerosolized prostaglandin I2 commenced), vasopressor dosages were not different (Table 3). The incidence of hypotension (mean arterial blood pressure < 65 mm Hg) was not different before (29%) or after initiation (28%) and had no impact on the magnitude of improvement in PaO2/FIO2 (29 ± 44 vs 34 ± 52 mm Hg, respectively; P = .59). In the multivariate logistic regression model results, mean arterial blood pressure, vasopressor use, and number of agents used did not determine the responder rate (see below).
Vasopressor and Inotropic Dosages Before and After Initiation of Aerosolized Prostaglandin I2 Therapy
Oxygenation and Ventilation Response to Aerosolized Prostaglandin I2
There were no differences between pre- and post-aerosolized prostaglandin I2 measurements of FIO2, PEEP, VT, and mean arterial blood pressure (Table 4). Oxygenation improved markedly after the introduction of aerosolized prostaglandin I2, with mean PaO2/FIO2 increasing by 33 mm Hg (42%) for the entire sample and by 56 mm Hg (80%) when only responders were considered. A modest reduction was observed in both PaCO2 and end-tidal carbon dioxide pressure that coincided with a significant, but negligible, increase in V̇E, yet the relative change in V̇E (ie, corrected to a PaCO2 of 40 mm Hg) was not different. The mean time between pre- and post-arterial blood gas measurements was 2.2 ± 2.0h. Sixty-seven percent of the pre-/post-arterial blood gas measurements were done within 2 h, and 89% were done within 4 h.
Gas Exchange and Ventilator Variables Before and After Initiation of Aerosolized Prostaglandin I2 Therapy
Impact of Baseline Oxygenation on the Response to Aerosolized Prostaglandin I2
Those with the least impaired oxygenation (highest group), had a significantly greater improvement in PaO2/FIO2 (51 ± 63 mm Hg) than those in either the intermediate (34 ± 46 mm Hg, P = .002) or lowest group (19 ± 37 mm Hg, P < .001) (Fig. 2). When only responders were considered, the increase in PaO2/FIO2 was 70 ± 49 (highest group), 58 ± 46, (intermediate group) and 39 ± 45 for (lowest group). Among responders, when compared with the lowest group, the magnitude of increase in PaO2/FIO2 was significantly greater in both the intermediate (P = .039) and highest groups (P = .005).
Mean ratio of PaO2/FIO2 before and after the initiation of aerosolized prostacyclin and the magnitude of change based on the severity of oxygenation defects in Group A (PaO2/FIO2 < 60 mm Hg), Group B (PaO2/FIO2 60–90 mm Hg), and Group C (PaO2/FIO2 > 90 mm Hg).
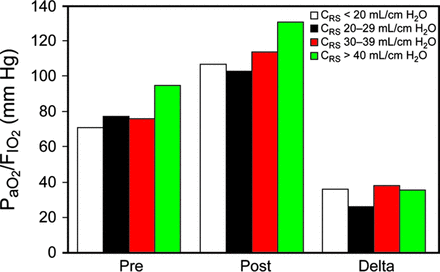
Impact of Baseline CRS on the Response to Aerosolized Prostaglandin I2
All four CRS groups also had a significant improvement (P < .001) in oxygenation with aerosolized prostaglandin I2 (Fig. 3). Unlike the oxygenation groups, however, there was not a steadily increasing improvement in the magnitude of PaO2/FIO2 response, since CRS increased whether all subjects (P = .27) or only responders (P = .58) were considered. This was readily apparent when comparing the columns marked “Delta” between Figure 2 and Figure 3. Nonetheless, the number of aerosolized prostaglandin I2 responders tended to increase as CRS improved: 53% (CRS < 20 mL/cm H2O), 56% (CRS = 20–29 mL/cm H2O), 75% (CRS = 30–39 mL/cm H2O), and 68% (CRS ≥ 40 mL/cm H2O). Among responders, there was an impressive increase in PaO2/FIO2: 67 ± 64 (CRS < 20 mL/cm H2O), 49 ± 37, (CRS = 20–29 mL/cm H2O), 53 ± 49 (CRS = 30–39 mL/cm H2O), and 62 ± 49 mm Hg (CRS ≥ 40 mL/cm H2O). Significant differences were observed between CRS groups: CRS < 20 versus CRS = 30–39 mL/cm H2O (P = .009); CRS = 20–29 versus CRS = 30–39 mL/cm H2O (P = .02).
Mean PaO2/FIO2 before and after the initiation of aerosolized prostacyclin and the magnitude of change based on groupings according to derangements in compliance of the respiratory system (CRS).
ARDS Etiology and the Response to Aerosolized Prostaglandin I2
Regardless of ARDS etiology, PaO2/FIO2 increased significantly in response to aerosolized prostaglandin I2 (Table 5). Subjects with trauma-associated ARDS had the largest increase in PaO2/FIO2, which was significantly greater compared with those with ARDS associated with either pneumonia or sepsis. Although the baseline PaO2/FIO2 also was higher in subjects with trauma-associated ARDS, the differences between etiologies in baseline PaO2/FIO2 was not significant (P = .28).
Oxygenation Response to Aerosolized Prostaglandin I2 Based on Etiology of ARDS
Subjects with sepsis as the primary source of ARDS had both the smallest increase in PaO2/FIO2 and lowest responder rate. All subjects who had sepsis as a contributing factor in the development of ARDS (n = 68) were compared with those without sepsis (n = 139). Despite having a similar baseline oxygenation, those with sepsis had a significantly smaller improvement in PaO2/FIO2 in response to aerosolized prostaglandin I2 than those without sepsis (18 ± 35 vs 40 ± 55, P = .02) (Fig. 4). Subjects with sepsis as a primary or secondary source of ARDS also tended to have a lower responder rate compared with those without sepsis (55% vs 65%), although this was not significant (odds ratio 0.78 [95% CI 0.55–1.11], P = .23).
Mean PaO2/FIO2 before and after the initiation of aerosolized prostacyclin and the magnitude of change based on the presence or absence of sepsis.
Direct and indirect sources of ARDS each accounted for 37% of the sample, whereas mixed mechanisms accounted for 26%. Regardless of etiology, all groups experienced significant increases in PaO2/FIO2 in response to aerosolized prostaglandin I2 (P < .001). The magnitude of improvement in PaO2/FIO2 for direct, indirect, and mixed was 34 ± 51, 22 ± 45, and 41 ± 51 mm Hg, respectively, and was significant only between indirect and mixed mechanisms (P = .03). In addition, the respective responder rate was 64, 55, and 67% and was not statistically significant.
Impact of ARDS Duration Before Commencing Aerosolized Prostaglandin I2
In the majority of subjects (55%), aerosolized prostaglandin I2 was initiated on the day of ARDS onset, and 82% of initiations occurred in the early phase of ARDS (ie, days 1–4). Initiation during either the early or later phases of ARDS was associated with significant improvements in PaO2/FIO2 (77 ± 37 vs 111 ± 69 mm Hg, P < .001; 82 ± 39 vs 109 ± 57 mm Hg, P < .001, respectively). Also, the magnitude of PaO2/FIO2 improvement was not different between ARDS phases (34 ± 53 mm Hg vs 27 ± 29 mm Hg, respectively, P = .96).
Multivariate Regression Model
Most variables of interest included in the initial model failed to predict aerosolized prostaglandin I2 responders. Of particular interest, factors such as nebulizer type (mini-Heart vs Aerogen), baseline PEEP, VT, ARDS etiology, injury mechanism (ie, direct, indirect, mixed), sepsis, mean arterial blood pressure, and vasopressor use fell out of the initial model. In the final model, aerosolized prostaglandin I2 responders were associated with baseline PaO2/FIO2, CRS, lower APACHE II score, and absence of neuromuscular blockade (Table 6). For every 10 mm Hg increase in baseline PaO2/FIO2, the odds of responding to aerosolized prostaglandin I2 increased by 10%, whereas for every 1-mL/cm H2O increase in baseline CRS, the odds of responding increased by 4%. For every 1-point increase in APACHE II measured at ARDS onset, the odds of responding to aerosolized prostaglandin I2 diminished by 7%. Although only approaching statistical significance, the absence of neuromuscular blockade also decreased the odds of responding by 42%.
Adjusted Analysis of Aerosolized Prostaglandin I2 Response as a Function of Baseline PaO2/FIO2 and Compliance of the Respiratory System
Discussion
Inhaled pulmonary vasodilators commonly are used for treating refractory hypoxemia despite limited data indicating precisely which ARDS patients may benefit. In the present study, approximately 60% of subjects had significantly improved oxygenation with aerosolized prostaglandin I2. Moreover, we demonstrated that 2 of the classic signifiers of FRC in ARDS, baseline oxygenation (as measured by PaO2/FIO2) and CRS, are the most salient determinants of a positive response to aerosolized prostaglandin I2. The magnitude of improvement in PaO2/FIO2 in our ARDS subjects was greater than that found in some studies of ARDS (ΔPaO2/FIO2 of 10–21 mm Hg),7,16,24,27–29 but was consistent with others (28–44 mm Hg).6,30–32 In addition, those with least impaired oxygenation had a significantly greater improvement in PaO2/FIO2 and a substantially higher responder rate than those with more severely impaired baseline oxygenation. Although the CRS groups did not demonstrate the same pattern of a proportional increase in the magnitude of oxygenation improvement, nonetheless there was a distinct increase in the response rate to aerosolized prostaglandin I2 as CRS improved.
Our findings support the study hypothesis that the effectiveness of aerosolized prostaglandin I2 is dependent upon the amount of aerated lung parenchyma, signified by FRC. They are also in accord with the classic studies on lung mechanics and gas exchange in ARDS demonstrating that oxygenation efficiency and CRS are directly related to FRC.33,34 From this, it follows that the effectiveness of aerosolized prostaglandin I2 might be enhanced when combined with lung recruitment strategies, such as higher PEEP, prone positioning, and/or recruitment maneuvers. A review of prone positioning cited several studies where combining inhaled NO with prone position had an additive effect on improving oxygenation.35 Therefore, when initiation of aerosolized prostaglandin I2 therapy fails to improve oxygenation sufficiently, clinicians might consider additional therapies (eg, prone position, higher PEEP, or recruitment maneuvers) that may enhance the effects of either aerosolized prostaglandin I2 or inhaled NO in very severe cases of ARDS.
Our subjects with sepsis had smaller improvement in PaO2/FIO2 and a tendency toward a lower response rate to aerosolized prostaglandin I2. Sepsis causes endothelial dysfunction, leading to deregulated release of both nitric oxide and prostacyclin.36 Therefore, our results are consistent with other studies that found that inhaled NO is less effective in improving gas exchange in sepsis-associated ARDS.14,15 This appears to be a novel finding, since we are unaware of any aerosolized prostaglandin I2 study reporting diminished response in sepsis-associated ARDS. In contrast, subjects with trauma-associated ARDS tended to have both a greater improvement in PaO2/FIO2 and response rate. This also appears to be a novel finding, though it shares similarities with other studies. Trauma-associated ARDS is distinct from other etiologies in terms of often having both a less severe clinical course and lower systemic inflammatory response37 as well as lower dead-space ventilation38 and lower mortality.39,40
Both the magnitude of improvement and response rate of PaO2/FIO2 to aerosolized prostaglandin I2 were not different when subjects were classified as having either direct or indirect injury. This is in contrast to the findings of Domenighetti et al,16 who found that aerosolized prostaglandin I2 was only effective in those with indirect injury. However, only 14 subjects were studied. Given our findings that approximately 40% of subjects with ARDS are non-responders, this may only reflect an artifact from a small sample size.
A meta-analysis41 of aerosolized prostaglandin I2 in ARDS concluded that there was insufficient evidence supporting the routine use of aerosolized prostaglandin I2, because there is no indication that it improves outcomes despite improving oxygenation. However, complex phenomena, such as determinants of mortality in ARDS, are not necessarily amenable to a single therapy. Aerosolized prostaglandin I2 might be useful in concert with other therapies targeting a specific goal known to impact mortality. In the absence of demonstrable harm or excessive cost/benefit ratio, there is a justifiable rationale to pursue using inhaled vasodilators in subsets of ARDS described below.
There is persuasive evidence that cor pulmonale is associated with mortality in ARDS and is present in approximately 50% of severe cases.42–44 Also, preclinical research has demonstrated the additive effects of high-stretch tidal ventilation and hyperoxia in promoting ventilator-induced lung injury.45 Given the heterogeneous nature of lung injury and maldistribution of VT despite achieving lung-protective goals,46 it remains plausible that prolonged, regional exposure to both excessive stretch and hyperoxia may impact outcomes in ways not yet appreciated. The ability of aerosolized prostaglandin I2 to improve oxygenation and reduce right-ventricular afterload at a less toxic FIO2 might yet be shown to improve outcomes when incorporated into a multitargeted approach. In the interim, we would suggest that aerosolized prostaglandin I2 be considered in these situations, particularly when right-heart dysfunction is suspected or demonstrated by echocardiography.
The majority of our subjects (68%) were studied within 48 h of ARDS onset, with 83% of all subjects meeting severe ARDS criteria at the time aerosolized prostaglandin I2 therapy commenced. However, our mortality was higher than the ranges reported both by the Berlin Definition Study Group18 and the more recent LUNG SAFE Investigators47 (56% vs 42–48% and 42–50%, respectively). Direct comparisons between our cohort and these studies are problematic because the latter were based on classifications determined on the day of ARDS onset. Moreover, our subjects were a distinct subset of severe ARDS that could be classified as non-responders to traditional therapy, hence the need for ancillary strategies traditionally considered to be salvage or rescue therapies.
The limitations of our study stem from its retrospective nature and its being based upon a single center. However, our study is by far the largest ever done on aerosolized prostaglandin I2 in ARDS with diverse, well-represented etiologies, as well as having subjects of diverse racial/ethnic backgrounds. It also was done predominantly in highly unstable subjects early in the course of severe ARDS. These represent the cohort of ARDS patients most in need of effective therapies to stabilize gas exchange and in whom evidence suggests that other therapies (higher PEEP,48 prone positioning,49 recruitment maneuvers,50 and neuromuscular blockade51) improve outcomes. Our results, therefore, provide uniquely detailed information that might inform the design of a future trial to assess whether aerosolized prostaglandin I2 might improve outcomes in a highly circumspect subset of ARDS.
Conclusions
In summary, aerosolized prostaglandin I2 improved oxygenation in approximately 60% of subjects presenting with moderately severe or severe ARDS. Its efficacy was apparent regardless of the severity of impairment in baseline oxygenation or CRS; subgroupings of ARDS based upon etiology, early versus late, or direct versus indirect injury; or type of aerosol delivery system used. Its effectiveness also appears to be higher in those with less severely impaired oxygenation and CRS. This only suggests that the effectiveness of aerosolized prostaglandin I2 is dependent upon FRC and therefore may be improved when used together with strategies that improve FRC (eg, prone positioning, recruitment maneuvers, and high PEEP).
Acknowledgments
We acknowledge the diligence of our respiratory therapists, who are responsible for executing aerosolized prostaglandin I2 therapy and closely monitoring its effects. Without their dedication and high degree of professionalism, this study would not have been possible.
Footnotes
- Correspondence: Richard H Kallet MSc RRT FAARC, Respiratory Care Services, Department of Anesthesia and Perioperative Care, Zuckerberg San Francisco General Hospital and Trauma Center, Building 5: GA-2, 1001 Potrero Avenue, San Francisco, CA 94110. E-mail: rich.kallet{at}ucsf.edu.
The authors have disclosed no conflicts of interest.
Mr Burns presented a version of this paper as an Editors' Choice abstract at AARC Congress 2016, held October 15–18, 2016, in San Antonio, Texas.
See the Related Editorial on Page 1113
- Copyright © 2017 by Daedalus Enterprises