Abstract
BACKGROUND: Although effective in the neonatal population, exogenous pulmonary surfactant has not demonstrated a benefit in pediatric and adult subjects with hypoxic lung injury despite a sound physiologic rationale. Importantly, neonatal surfactant replacement therapy is administered in conjunction with low fractional FIO2 while pediatric/adult therapy is administered with high FIO2. We suspected a connection between FIO2 and surfactant performance. Therefore, we sought to assess a possible mechanism by which the activity of pulmonary surfactant is adversely affected by direct oxygen exposure in in vitro experiments.
METHODS: The mechanical performance of pulmonary surfactant was evaluated using 2 methods. First, Langmuir-Wilhelmy balance was utilized to study the reduction in surface area (δA) of surfactant to achieve a low bound value of surface tension after repeated compression and expansion cycles. Second, dynamic light scattering was utilized to measure the size of pulmonary surfactant particles in aqueous suspension. For both experiments, comparisons were made between surfactant exposed to 21% and 100% oxygen.
RESULTS: The δA of surfactant was 21.1 ± 2.0% and 35.8 ± 2.0% during exposure to 21% and 100% oxygen, respectively (P = .02). Furthermore, dynamic light-scattering experiments revealed a micelle diameter of 336.0 ± 12.5 μm and 280.2 ± 11.0 μm in 21% and 100% oxygen, respectively (P < .001), corresponding to a ∼16% decrease in micelle diameter following exposure to 100% oxygen.
CONCLUSIONS: The characteristics of pulmonary surfactant were adversely affected by short-term exposure to oxygen. Specifically, surface tension studies revealed that short-term exposure of surfactant film to high concentrations of oxygen expedited the frangibility of pulmonary surfactant, as shown with the δA. This suggests that reductions in pulmonary compliance and associated adverse effects could begin to take effect in a very short period of time. If these findings can be demonstrated in vivo, a role for reduced FIO2 during exogenous surfactant delivery may have a clinical benefit.
Introduction
Human metabolism relies primarily on an aerobic process for the combustion of nutrients and extraction of energy at the cellular level. The process consumes oxygen and generates carbon dioxide. In healthy subjects, ambient concentrations of oxygen (21%) are sufficient to sustain metabolism. However, a number of pathophysiologic processes can impede exchange of oxygen from the lungs into the blood, and therefore administration of high concentrations of oxygen is required to maintain adequate arterial oxygen levels and tissue oxygenation. Although sometimes clinically indicated, high oxygen concentrations may lead to oxygen toxicity. In vivo research suggests this toxicity may manifest as changes in airways responsiveness, inflammation, increased lung volumes, and depletion of type II pneumocyte proliferation.1–3
Pulmonary surfactant has been the subject of extensive research since its successful administration in newborn infants with respiratory distress syndrome was described by Fujiwara et al4 in the early 1980s. Since that time, its role in the care of premature infants as well as chemical constituents and specific function have been well described.5 More recently, the role of exogenous surfactant in the care of adult and pediatric subjects with ARDS has been investigated.6–9 Pathogenesis of ARDS has been partly attributed to alterations in endogenous surfactant.10 The physiologic rationale for the administration of surfactant in this population is to restore surfactant function, thereby improving pulmonary system compliance and oxygenation. Despite promising data in pilot trials, large controlled investigations have not demonstrated a clear benefit of exogenous surfactant in subjects with ARDS as it had little or no effect on oxygenation or long-term outcomes.7,11 However, since the majority of exogenous surfactant protocols in pediatric and adult patients call for high concentrations of inspired oxygen during therapy (which is not the case in the neonatal population), we suspected that there may be a connection between oxygen concentration and surfactant performance.
Furthermore, most research has focused on the cellular mechanisms of oxygen toxicity, but the effects of direct high-concentration oxygen on surfactant mechanical properties have not been adequately described in a controlled laboratory investigation. Oxygen toxicity, which results from the delivery of increased concentrations of oxygen, has been associated with absorption atelectasis, pulmonary edema and lung fibrosis.12–15 Oxygen toxicity is compounded by increased FIO2 as well as duration of exposure. Typically, FIO2 ≤ 0.50 has been recommended as a therapeutic target to reduce the likelihood of oxygen toxicity in human subjects.16
A biochemical mechanism, as the basis for oxygen toxicity, has been proposed; it results from the cellular production of partly reduced oxygen metabolites.17,18 Indeed, lung injury may be exacerbated by an oxygen free radical process that results in cellular damage and impeded gas exchange.19–22 In vivo translational research has demonstrated that administration of an oxygen free radical-fighting molecule, superoxide dismutase, prevents many deleterious effects of breathing high-concentration oxygen in an animal model.23 However, survival rates, morphologic changes, and histologic findings were not completely preserved, suggesting mechanisms other than those mediated by oxygen free radicals may contribute to lung injury.
The present research group has previously demonstrated that in excised lungs, exposure to different oxygen concentrations can have near-immediate effects on mechanical behavior. That is to say, lungs inflated with 100% oxygen have significantly greater stiffness and hysteresis than those exposed to air. This effect was most apparent at low lung pressures (∼4 cm H2O) and smaller at much higher pressures (∼20 cm H2O).24 Mechanical responses at low pressures are known to be dominated by surfactant behavior, and we hypothesized that the gas composition was directly affecting this substance.
It should be noted that experiments by Wildebeer-Venema25 showed that exposure to different gases had little to no effect on the static minimum surface tension of surfactant. However, that study did not explore dynamic surface tension and re-spreading of surfactant, which is very important in vivo because the surface area expands and contracts inside the alveoli with breathing. Therefore, we sought to describe the performance of isolated pulmonary surfactant at high oxygen concentration (100%) and low oxygen concentration (air, 21%) through the careful implementation of 2 distinct experiments: 1) on the performance (directly related to ability of surfactant to redistribute), and 2) on the suspension structure of surfactant.
QUICK LOOK
Current knowledge
Exogenous surfactant administration is effective in the neonatal population with surfactant deficiency, but is not effective for pediatric and adult patients with ARDS despite data showing that surfactant function is allayed. We suspected a connection between oxygen concentration and surfactant performance.
What this paper contributes to our knowledge
The characteristics of pulmonary surfactant were adversely affected by short-term exposure to oxygen. Specifically, surface tension studies revealed that short-term exposure of surfactant film to high concentrations of oxygen expedited the frangibility of pulmonary surfactant, as shown with the surface area change. These data suggest that reductions in pulmonary compliance and associated adverse effects could begin to take effect in a very short period of time.
Methods
Surfactant Preparation
The surfactant preparation utilized for the experiments was Infasurf (ONY, Amherst, New York), a bovine, nonpyrogenic pulmonary surfactant. It is a natural calf lung surfactant extract that includes phospholipids, mainly dipalmitoylphosphatidylcholine, neutral lipids, and hydrophobic surfactant-associated proteins B and C, and is cleared for human use by the FDA. According to established laboratory protocol, the surfactant was suspended in chloroform-anhydrous (Sigma-Aldrich, St. Louis, Missouri) to a concentration of 1 mg/mL and stored in a temperature-regulated refrigerator (∼7°C).
Surface Tension Measurement
Surface tension studies were performed using a Langmuir-Wilhelmy balance (Micro Trough X, Kibron, Helsinki, Finland) with a surface area of ∼125 cm2. The technique has been used previously to characterize pulmonary surfactant in vitro.26,27 Fundamentally, the balance is used to quantify surface tension through the ability of the surfactant to redistribute across the surface area of the device after changing the surface area. This property is associated with substance frangibility, and in the context of surfactant, its ability to reduce surface tension and increase compliance in vivo.27 Preceding the experiments, the equipment was calibrated according to the manufacturer's specifications. Preparation of the instrument included deposition of a 50 μL of pure water onto the balance plate using a micro-syringe. The water layer serves as the subphase, upon which the surfactant is deposited. After being warmed to ambient temperature, 20 μL of surfactant was deposited on the water subphase. An illustration of the experimental setup is shown in Figure 1. The entire device was enclosed in a humidity- and temperature-controlled environmental enclosure (depicted in Fig. 1). The relative humidity was maintained at ∼80% and room temperature (∼21°C). The surfactant surface was repeatedly compressed with the device (ie, cycled). The device was cycled between lower and upper bounds of surface tension γ: 15 mN/m and 45 mN/m respectively, at 93.5 mm/min (this corresponds to a nominal 50% area change/min). After a typical Langmuir-Wilhelmy cycle (see Fig. 2), the γ-area curve shifts to the left; this is because surfactant molecules are lost to the aqueous subphase, and thus slightly greater compression is required to achieve the programmed lower bound of γ. Our experiments aimed to measure the effect of oxygen concentration on this shift. Two distinct experimental procedures were conducted: in air, and in 100% oxygen. To introduce oxygen into the system, pure oxygen was introduced into the enclosure at a flow of 3 L/min. The oxygen concentration was continuously monitored during the experiments with the Oxygen-Meter 200 (Apogee Instruments, Logan, Utah). Each experiment comprised 30 cycles. A total of 3 experiments were conducted in 21% oxygen, and 6 in 100% oxygen. Surface tension data were continuously recorded utilizing FilmWare (version 3.57, Kibron). In summary, 2 distinct experiments were conducted on surfactant: 1) in air, and 2) in 100% oxygen.
Illustration of experimental setup with the Langmuir-Wilhelmy balance.
Plot of the surface tension (γ) and area. The area is the surface area that the surfactant occupied on the Langmuir-Wilhelmy balance (expressed as a percentage of total) in A: 21% oxygen and B: 100% oxygen. C: Plot of surface area change (δA) between 21% and 100% oxygen. Horizontal lines represent the mean and whiskers denote ± SD. δA is the change in area expressed as a percentage.
Calculation of Difference in Surface Area
For each 30-cycle experiment, the aforementioned shift (expressed as a percentage, δA) was calculated from the first to last cycle. A smaller δA indicates a greater ability of surfactant to dynamically redistribute. An illustration of the calculation of the δA is shown in Figure 2.
Surfactant Micelle Size Measurement Using Dynamic Light Scattering
A surfactant suspension was prepared with water in 50-mL glass beakers at a ratio of 3 μL surfactant to 2 mL water. The preparation was mixed using a magnetic stirrer, according to standard laboratory protocol. The use of a magnetic stirrer ensures that the preparation is thoroughly but gently mixed without disturbing the surfactant to the point of foaming. Four iterations were performed: 1) surfactant suspension and introduction of 2 L/min air, 2) surfactant suspension and introduction of 2 L/min 100% oxygen, 3) water-only control and 2 L/min air, and 4) water-only control and 2 L/min 100% oxygen. In all cases, the gases were introduced for a total of 3 h. Each beaker was then emptied into a measurement cuvette. The surfactant particles formed varying-sized micelles. The size of these micelles was measured using a dynamic light-scattering device (NanoBrook 90 Plus, Brookhaven Instruments, Holtsville, New York). Dynamic light scattering is a technique used to quantify the size of small particles in suspension, and it has been used widely in chemistry and biology.28,29 Measurements were repeated 10 times for each the air and the 100% oxygen preparation.
Statistical Analyses
Data were imported into Excel (version 14.6.7, Microsoft, Redmond, Washington) and Prism (version 6.0g, GraphPad Software, San Diego, California). The δA of surfactant in air and oxygen were compared using a Mann-Whitney U test. The measured micelle size data (from dynamic light scattering) were compared using an unpaired t test.
Results
Surface Tension Measurement
We observed a statistically significant difference between the δA of surfactant during exposure to 21% oxygen and 100% oxygen (21.1 ± 2.0% vs 35.8 ± 2.0%, P = .02) (see Fig. 2). Given the fact, that, in oxygen, a greater surface area change is required to achieve the target lower surface tension, more surfactant is lost to the aqueous subphase, and we attribute that to increased frangibility.
Surfactant Micelle Diameter Measurement Using Dynamic Light Scattering
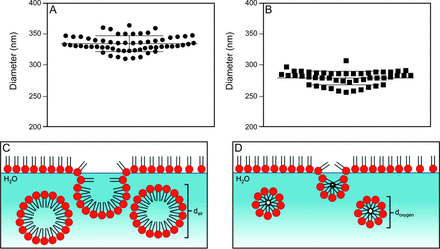
Dynamic light-scattering experiments revealed a micelle diameter of 336.0 ± 12.5 μm and 280.2 ± 11.0 μm in 21% and 100% oxygen, respectively (P < .001); corresponding to a ∼16% decrease in micelle diameter following exposure to 100% oxygen (Fig. 3).
Results from the dynamic light-scattering experiments. The diameter of the surfactant micelles from each sample are shown in A: 21% oxygen, and B: 100% oxygen. Micelle diameter was significantly smaller in 100% oxygen vs 21% oxygen (280.2 ± 11.0 μm vs 336.0 ± 12.5 μm, P <.001.). Horizontal bars represent the mean and whiskers show the SD of the entire sample. In C (21% oxygen) and D (100% oxygen), graphic depictions of the micelle diameter are shown to illustrate the measurements (surfactant particle size not drawn to scale, for illustration only).
Discussion
In an in vitro model, we demonstrated a statistically significant difference in the area change during controlled cycling of pulmonary surfactant, suggesting that pulmonary surfactant performance is negatively affected by exposure to high oxygen concentrations. As depicted in Figure 2, when surfactant is exposed to pure oxygen, a more pronounced reduction in surface area necessary to achieve a lower surface tension γ is observed. This indicates a lower surface density of available surfactant molecules on the aqueous surface. This occurs as surfactant molecules are lost within the aqueous subphase following repeated cycling in oxygen. It is conceivable that this loss is because of increased frangibility of the surfactant in 100% oxygen.
Under compression, molecule chains are driven into the aqueous subphase and curl because of the hydrophobic and hydrophilic nature of the surfactant molecules. Ideally, this curl would be tolerated and upon re-expansion, a majority of molecules would return to the surface. However, at a particular limit, the curled portion breaks off and forms a micelle (Fig. 3). We hypothesize that 100% oxygen reduces this limit, and allows the surfactant to break off more easily under compression. This assertion is consistent with our results from dynamic light scattering, where statistically significant differences in micelle diameter were observed. The smaller suspended particles (observed in 100% oxygen) would result from a more frangible surfactant.
Importantly, our data suggest that pulmonary surfactant performance is negatively affected by exposure to high oxygen concentrations. Surfactant supplementation has not been shown to produce a sustained or significant positive impact in patients with hypoxic lung injury.7 The results of the present work demonstrate the rapid deterioration in mechanical performance of surfactant as a result of high O2 exposure. This may offer a partial explanation as to why supplemental surfactant has not produced significant improvements in oxygenation and long-term outcomes in patients with lung injury.
There are important limitations to the present work that should be noted. First, instead of human derived surfactant, isolated bovine surfactant was used. However, bovine surfactant is analogous to human pulmonary surfactant and is approved in many countries for surfactant replacement therapy. Second, the environmental factors were not identical to those found within the lungs. The equipment and experimental conditions precluded exact temperature and humidity, but efforts were made to ensure that humidity was added to the environment. Preliminary experiments with surfactant with the equipment revealed that it evaporated at temperatures ∼37°C, which would significantly confound the surface tension and area measurements, and we therefore decided to limit the temperature to reduce this risk. Third, the colloid preparation utilized deionized water and not saline. However, since the protocol followed precedent, it's unlikely that this would have a significant confounding effect on the results. Fourth, our experiment was designed such that only 2 FIO2 concentrations were investigated. This limited our ability to determine if a critical threshold for FIO2 exists below a FIO2 of 1.0. In the future, it will be important to investigate a range of FIO2 to assess whether a concentration-dependent adverse effect on surfactant exists. Last, all experiments were conducted in vitro. However, surface tension and micelle diameter measurements are not possible to obtain in vivo with presently available equipment.
It is important to note that exogenous surfactant has been targeted in conditions with distinct underlying pathophysiologic processes. In terms of surfactant function, respiratory distress syndrome in neonatal patients can be characterized as a surfactant deficiency and acute lung injury, or ARDS can be characterized as a surfactant dysfunction.30–33 Factors such as airway edema, fibrosis, infection, and extrinsic airway pressures may contribute to surfactant dysfunction during ARDS, but could not be included in the present model. Since it is outside the scope of the present work, we cannot speculate on what the compounded effect of high-flow oxygen therapy and other factors would be upon surfactant mechanics, and further work is indicated in this area. Interestingly, neonatal patients typically receive lower FIO2 (0.21–0.30) as a function of targeting a low SpO2 for the prevention of retinopathy of prematurity as well as preventing development of bronchopulmonary dysplasia, whereas pediatric and adult patients with acute lung injury may receive FIO2 0.4–1.0, particularly around times associated with care.34,35 Despite the differences in pathophysiology, we questioned whether the FIO2 around the time of surfactant administration could affect the properties of surfactant, which, in turn could manifest as important clinical differences. Our data do indeed suggest a deterioration of surfactant function in high-oxygen concentration environments as evidenced by the increased frangibility of the molecules.
Future research should seek to assess the reversibility of oxygen's adverse effect on pulmonary surfactant through in vitro studies. Further, since we tested only 2 distinct oxygen concentrations, a specific concentration at which the negative effects of oxygen are not observed is not known. Work that assesses surfactant function over a wide range of oxygen concentrations may help to identify a specific level, below which surfactant is only minimally adversely affected. Further, in vivo work assessing the combined effects of factors known to cause surfactant dysfunction and induce lung injury with high oxygen concentrations should be completed.
Conclusions
Surface tension studies revealed that short-term exposure of surfactant film to high concentrations of oxygen expedited the frangibility of pulmonary surfactant, as shown with the altered surface area required to achieve a minimum surface tension value. This interpretation was consistent with mechanistically distinct light-scattering experiments on aqueous surfactant suspensions. This may suggest that reductions in pulmonary compliance and associated adverse effects could begin to take effect in a very short period of time. If these findings can be demonstrated in vivo, then a role for reduced FIO2 during exogenous surfactant delivery may be indicated and could enhance clinical benefit.
Acknowledgments
The authors acknowledge Dr Heather Clark of Pharmaceutical Sciences at Northeastern University for usage of the light scattering equipment, as well as Fulden Buyukozturk, Selena Di Maio, and Dr Rebecca Carrier for helpful guidance on the light scattering experiments.
Footnotes
- Correspondence: Andrew Gouldstone PhD, College of Engineering, Department of Mechanical and Industrial Engineering, Northeastern University, Boston, MA 02115. E-mail: a.gouldstone{at}northeastern.edu.
The authors have disclosed no conflicts of interest.
- Copyright © 2017 by Daedalus Enterprises