Abstract
BACKGROUND: The 6-min walk test (6MWT) is commonly performed to assess functional status in patients with chronic thromboembolic pulmonary hypertension. However, changes in heart rate and oxygen saturation (SpO2) patterns during 6MWT in patients with chronic thromboembolic pulmonary hypertension remain unclear.
METHODS: Thirty-one subjects with chronic thromboembolic pulmonary hypertension were retrospectively evaluated to examine the relationships between the change in heart rate (Δheart rate), heart rate acceleration time, slope of heart rate acceleration, heart rate recovery during the first minute after 6MWT (HRR1), change in SpO2 (ΔSpO2), SpO2 reduction time, and SpO2 recovery time during 6MWT, and the severity of pulmonary hemodynamics assessed by right heart catheterization and echocardiography.
RESULTS: Subjects with severe chronic thromboembolic pulmonary hypertension had significantly longer heart rate acceleration time (144.9 ± 63.9 s vs 96.0 ± 42.5 s, P = .033), lower Δheart rate (47.4 ± 16.9 vs 61.8 ± 13.6 beats, P = .02), and lower HRR1 (13.3 ± 9.0 beats vs 27.1 ± 9.2 beats, P < .001) compared to subjects with mild chronic thromboembolic pulmonary hypertension. Subjects with severe chronic thromboembolic pulmonary hypertension also had significantly longer SpO2 reduction time (178.3 ± 70.3 s vs 134.3 ± 58.4 s, P = .03) and SpO2 recovery time (107.6 ± 35.3 s vs 69.8 ± 32.7 s, P = .004) than did subjects with mild chronic thromboembolic pulmonary hypertension. Multivariate linear regression analysis showed only mean pulmonary arterial pressure independently was associated with heart rate acceleration time and slope of heart rate acceleration.
CONCLUSIONS: Heart rate and SpO2 change patterns during 6MWT are predominantly associated with pulmonary hemodynamics in subjects with chronic thromboembolic pulmonary hypertension. Evaluating heart rate and SpO2 change patterns during 6MWT may serve as a safe and convenient way to follow the change in pulmonary hemodynamics.
- six-minute walk test
- chronic thromboembolic pulmonary hypertension
- heart rate
- oxygen saturation
- pulmonary hemodynamics
- disease severity
Introduction
Chronic thromboembolic pulmonary hypertension is caused by unresolved thromboembolism of the pulmonary arteries, leading to right heart failure and death.1–3 Impairment of exercise capacity due to limited pulmonary circulation, which induces right ventricular overload, restricted stroke volume, ventilation-perfusion mismatch, and exercise-induced hypoxemia, is an important feature of chronic thromboembolic pulmonary hypertension. In clinical settings, the 6-min walk test (6MWT) is routinely performed to assess the functional status of chronic thromboembolic pulmonary hypertension.4 Among the variables included in the 6MWT, the 6-min walk distance (6MWD) is a primary outcome that reflects hemodynamic severity, functional capacity, and survival in patients with chronic thromboembolic pulmonary hypertension, especially in clinical trials evaluating the response to medical treatments.5–7 However, the practical utility of other parameters such as heart-rate response or oxygen desaturation-resaturation patterns in each disease state of chronic thromboembolic pulmonary hypertension remains unclear.
Variables derived from the 6MWT (other than the 6MWD) have been investigated in subjects with chronic respiratory diseases. Abnormal heart rate recovery at 1 min (HRR1), which is the reduction in heart rate at 1 min after exercise, is known to be a strong predictor of pulmonary hypertension in idiopathic pulmonary fibrosis8 and clinical worsening in pulmonary arterial hypertension (PAH).9 Impairment in the heart-rate response during the 6MWT, also known as a chronotropic response, has been considered to predict 6MWD in idiopathic and nonidiopathic PAH.10 Furthermore, oxygen saturation (SpO2) change patterns during the 6MWT in subjects with COPD were reported to predict peak exercise and air flow limitations.11 Regarding subjects with chronic thromboembolic pulmonary hypertension, one recent report described that exercise-induced desaturation during the 6MWT was observed in operable chronic thromboembolic pulmonary hypertension before pulmonary endarterectomy (PEA). And that heart rate during the 6MWT reserve [(peak heart rate − resting heart rate)/(220 − age − resting heart-rate response)] was associated with pulmonary vascular resistance 1 y post PEA.12 Thus, additional parameters of the 6MWT, such as heart rate and SpO2 change patterns, might have the potential to reveal more precise physiological states of chronic thromboembolic pulmonary hypertension. However, the confounding factors of chronic thromboembolic pulmonary hypertension, in addition to its pathophysiology, should also be considered (eg, oxygen flow, pulmonary vasodilator, PEA, and deconditioning).
The purpose of our study was to evaluate the relationships between parameters concerning the heart-rate response and oxygen desaturation-resaturation patterns during the 6MWT and the severity of pulmonary hemodynamics in subjects with mild to severe chronic thromboembolic pulmonary hypertension. Additionally, we tested the hypothesis that variables of the 6MWT post-PEA in subjects with severe chronic thromboembolic pulmonary hypertension would return to levels similar to those in subjects with mild chronic thromboembolic pulmonary hypertension.
QUICK LOOK
Current knowledge
The 6-min walk test (6MWT) is routinely performed to assess the functional status of patients with chronic thromboembolic pulmonary hypertension. The 6-min walk distance (6MWD) is a primary outcome that reflects hemodynamic severity, functional capacity, and survival in patients with chronic thromboembolic pulmonary hypertension.
What this paper contributes to our knowledge
Heart rate acceleration and recovery time were slower and the slope of heart rate acceleration was less steep during 6MWT in subjects with severe chronic thromboembolic pulmonary hypertension than in those with mild chronic thromboembolic pulmonary hypertension. Moreover, SpO2 reduction time during 6MWT and recovery time after 6MWT were slower in subjects with severe chronic thromboembolic pulmonary hypertension than in those with mild chronic thromboembolic pulmonary hypertension.
Methods
Subject Selection
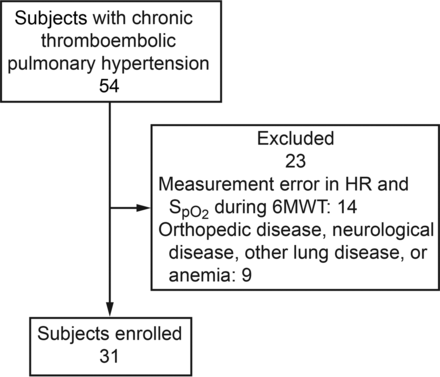
We retrospectively enrolled 54 subjects with chronic thromboembolic pulmonary hypertension who had undergone 6MWT, pulmonary function test, diagnostic right heart catheterization, and echocardiography at Chiba University Hospital in Chiba, Japan, between July 2011 and December 2016. Chronic thromboembolic pulmonary hypertension was defined as mean pulmonary arterial pressure ≥ 25 mm Hg with normal wedge pressure, as determined by subjects having undergone right heart catheterization with therapeutic anticoagulation for > 3 months.1 Lung perfusion scans that revealed segmental or larger defects concomitant with normal ventilation scans were also required for diagnosis. Findings suggestive of chronic thromboemboli were confirmed with pulmonary angiography.1,3 Subjects with measurement error in pulse rate and SpO2 during the 6MWT were excluded, as were subjects with orthopedic or neurological disorders, anemia, or active lung diseases other than chronic thromboembolic pulmonary hypertension. A total of 31 subjects were evaluated (Fig. 1). Subjects with chronic thromboembolic pulmonary hypertension were categorized according to disease severity (mild chronic thromboembolic pulmonary hypertension: mean pulmonary arterial pressure < 40 mm Hg; severe chronic thromboembolic pulmonary hypertension: mean pulmonary arterial pressure ≥ 40 mm Hg). To examine the effects of PEA, 10 of the 31 subjects who had all of the above data both pre- and post-PEA were separately evaluated. The study database was anonymized and complied according to the requirements of the Ministry of Health, Labor, and Welfare, which is dedicated to privacy, information technology, and civil rights in Japan. The Ethics Committee of Chiba University approved the study protocol (approval number 1259).
Flow chart. HR = heart rate, 6MWT = 6-min walk test.
Pretest Evaluations
All subjects had undergone baseline hemodynamic testing with right heart catheterization, including measurements of mean pulmonary arterial pressure, systolic pulmonary arterial pressure, diastolic pulmonary arterial pressure, pulmonary vascular resistance, PaO2, mixed venous partial pressure of oxygen (Pv̄O2), mixed venous oxygen saturation (Sv̄O2), cardiac index, cardiac output, and alveolar-arterial oxygen difference (P(A-a)O2). Doppler echocardiography using an AplioTM 300 ultrasound (Toshiba Medical, Tochigi, Japan) with a PST-25BT transducer (2.5 MHz; Toshiba Medical) was performed on all subjects at the end of expiration.13 Estimated systolic pulmonary arterial pressure, tricuspid regurgitation pressure gradient, and left ventricular ejection fraction (LVEF) were recorded.
Pulmonary function was assessed with a spirometer (CHSTAC-8900; Chest MI, Tokyo, Japan), and total lung volume and lung diffusion capacity for carbon monoxide (DLCO) were then measured per helium dilution and single-breath methods, respectively, according to the methods described in the American Thoracic Society's 1994 update.14
Subject Monitoring/Data Collection in 6MWT
The 6MWT was performed according to published guidelines.4 In the present study, 6MWT was performed in an indoor corridor (30 m in length) under quiet conditions. All subjects completed at least one 6MWT before data collection had begun in order to avoid learning effects.4 Ambulatory oxygen was permitted during the test if the subject was already on long-term oxygen therapy.
We recorded 6MWD. Pulse rate (PR) and SpO2 were measured for 2 min at baseline, continuously during the test, and 3 min for recovery by finger probe pulse oximeter (Pulsox-300i; Minolta, Tokyo, Japan). Since there were no subjects with obvious arrhythmia in the present study, PR was regarded as heart rate, as described in previous studies.8,9 Data were recorded every 1 s and were transferred to a personal computer. Methods for the calculation of heart rate parameters are shown in Figure 2A. Heart rate at rest and maximal heart rate were evaluated and Δheart rate was calculated (maximum heart rate–heart rate at rest). Heart rate acceleration time (time taken to increase to 75% of Δheart rate;) and slope of heart rate acceleration [slope of heart rate until point at which the subject has increased to 75% of change in heart rate; (75% of Δheart rate)/(heart rate acceleration time)] were also calculated. Heart rate recovery during the first minute after 6MWT (HRR1) was defined as the difference between a subject's heart rate at completion of 6MWT and at 1 min after completion of 6MWT.9 Methods for evaluating SpO2 parameters are shown in Figure 2B. SpO2 at rest, SpO2 after 6MWT, and lowest SpO2 were evaluated and ΔSpO2 was calculated (lowest SpO2 –SpO2 at rest). Further, SpO2 reduction time (time taken to decrease 75% of ΔSpO2) and SpO2 recovery time [time taken to recover to 75% of (SpO2 after 6MWT − SpO2 at rest)] were calculated. We also recorded the 10-point-modified Borg scale15 score after 6MWT. The 6MWT, right heart catheterization, echocardiography, and pulmonary function were measured during the same hospitalization for diagnosis and follow-up within 1–2 weeks.
Methods for calculation of pulse rate (A) and SpO2 (B) parameters during 6-min walk test (6MWT). A: ΔHR was defined as maximum HR − HR at rest. HR acceleration time was defined as the time taken to increase to 75% of ΔHR. Slope of HR acceleration was defined as 75% of ΔHR/HR acceleration time. HRR1 was defined as the difference between a subject's heart rate at completion of the 6MWT and at 1 min after completion of the 6MWT. B: Methods for calculation of SpO2 during 6MWT. ΔSpO2 was defined as lowest SpO2 − SpO2 at rest. SpO2 reduction time was defined as the time taken to decrease to 75% of ΔSpO2. SpO2 recovery time was defined as the time taken to recover to 75% of (SpO2 after 6MWT − SpO2 at rest). HR = heart rate, HRR1 = recovery during the first minute after 6MWT.
Statistical Analysis
Data are presented as mean ± SD. Differences in mean values between the 2 groups (ie, mild vs severe chronic thromboembolic pulmonary hypertension) were assessed with the Mann-Whitney U-test. Spearman's rank correlation coefficient was used to evaluate the relationship between the 6MWT parameters and age, physical parameters, right heart catheterization parameters, echocardiographic parameters, and pulmonary function parameters. Multiple linear regression analyses were performed to evaluate the independent association between the 6MWT parameters and right heart catheterization, echocardiographic, and pulmonary function parameters. In this analysis, clinical factors to which the 6MWT parameters were significantly correlated were included in the univariate regression analysis. In subjects who received PEA thereafter, intragroup comparisons (pre-PEA vs post-PEA) were made using the Wilcoxon signed-rank test for 6MWT parameters. The number of cases during the study period determined the sample size. Values of P < .05 were considered statistically significant. All analyses were carried out with the JMP10.0 software program (SAS institute, Cary, North Carolina).
Results
Subject Characteristics
Our study included 31 subjects with chronic thromboembolic pulmonary hypertension (World Health Organization functional class I, n = 3; class II, n = 17; class III, n = 10; class IV, n = 1).16 Baseline characteristics of subjects are shown in Table 1. Nineteen subjects (61.3%) had received long-term oxygen therapy. The mean prescribed oxygen flow was 2.3 L during exercise. No subjects were using β-blockers.
Baseline Characteristics of Subjects With Chronic Thromboembolic Pulmonary Hypertension
6MWT Parameters According to Severity of Chronic Thromboembolic Pulmonary Hypertension
Table 2 shows the 6MWT parameters for our sample of 31 subjects with chronic thromboembolic pulmonary hypertension. Compared with subjects with mild chronic thromboembolic pulmonary hypertension, subjects with severe chronic thromboembolic pulmonary hypertension had shorter 6MWD. Subjects with severe chronic thromboembolic pulmonary hypertension had significantly longer heart rate acceleration times (144.9 ± 63.9 s vs 96.0 ± 42.5 s, P = .033) and lower Δheart rate (47.4 ± 16.9 beats/min vs 61.8 ± 13.6 beats/min, P = .02), lower slope of heart rate acceleration (0.3 ± 0.1 vs 0.7 ± 0.4, P < .001), lower HRR1 (13.3 ± 9.0 beats vs 27.1 ± 9.2 beats, P < .001). Heart rate at rest tended to be higher in subjects with severe chronic thromboembolic pulmonary hypertension than in those with mild chronic thromboembolic pulmonary hypertension, but this difference was not significant (77.4 ± 11.4 beats/min vs 69.5 ± 7.1 beats/min, P = .08). Subjects with severe chronic thromboembolic pulmonary hypertension had significantly longer SpO2 reduction times (178.3 ± 70.3 s vs 134.3 ± 58.4 s, P = .025) and SpO2 recovery times (107.6 ± 35.3 s vs 69.8 ± 32.7 s, P = .004) than did subjects with mild chronic thromboembolic pulmonary hypertension.
Data for All Outcomes During 6MWT in Subjects With Chronic Thromboembolic Pulmonary Hypertension
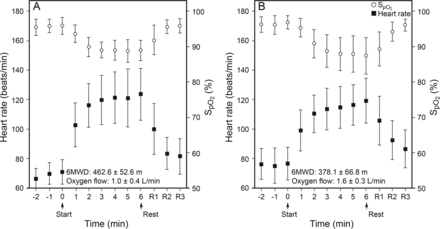
Figure 3 presents sequential mean changes of heart rate and SpO2 during the 6MWT for subjects with mild versus severe chronic thromboembolic pulmonary hypertension. In subjects with severe chronic thromboembolic pulmonary hypertension, heart rate increased slowly compared to those with mild chronic thromboembolic pulmonary hypertension. Subjects with severe chronic thromboembolic pulmonary hypertension continued desaturating throughout the 6MWT, while in subjects with mild chronic thromboembolic pulmonary hypertension the SpO2 decreased during the first 3 min and then remained stable during the 6MWT.
Sequential changes in heart rate and SpO2 during 6MWT (mean ± SD). In subjects with severe chronic thromboembolic pulmonary hypertension (B), heart rate increased slowly compared to subjects with mild chronic thromboembolic pulmonary hypertension (A). SpO2 decreased during the first 3 min and then remained stable during 6MWT in subjects with mild chronic thromboembolic pulmonary hypertension, while subjects with severe chronic thromboembolic pulmonary hypertension continued desaturating throughout 6MWT. HR = heart rate, 6MWT = 6-min walk test, 6MWD = 6-min walk distance.
Independent Associations of Severe Chronic Thromboembolic Pulmonary Hypertension in 6MWT Parameters
Correlations between the 6MWT parameters and clinical factors are shown in Table 3. Heart rate acceleration time was mainly positively correlated with mean pulmonary arterial pressure (r = 0.47, P = .008) and negatively correlated with CO (r = −0.41, P = .02). The slope of heart rate acceleration was mainly negatively correlated with mean pulmonary arterial pressure (r = −0.67, P < .001) and positively correlated with CO (r = 0.37, P = .041). The Δheart rate was mainly negatively correlated with mean pulmonary arterial pressure (r = −0.39, P = .032), although this correlation was weak. The 6MWD was mainly negatively correlated with mean pulmonary arterial pressure (r = −0.61, P < .001) and positively correlated with total lung capacity (r = 0.69, P < .001). HRR1 was mainly negatively correlated with mean pulmonary arterial pressure (r = −0.56, P = .001) and positively correlated with DLCO/VA (r = 0.47, P = .008). The SpO2 reduction time was mainly positively correlated with mean pulmonary arterial pressure (r = 0.43, P = .02) and negatively correlated with CO (r = −0.42, P = .02) and LVEF (r = −0.45, P = .01). The SpO2 recovery time was mainly positively correlated with mean pulmonary arterial pressure (r = 0.61, P < .001) and negatively correlated with DLCO/VA (r = −0.43, P = .02). Age was not significantly correlated with the 6MWT parameters.
Correlations Between Outcomes During 6MWT and Clinical Data in Subjects With Chronic Thromboembolic Pulmonary Hypertension
Multivariate linear regression analyses are shown in Table 4. The heart rate acceleration time and slope of heart rate acceleration were independently associated with mean pulmonary arterial pressure. HRR1 and SpO2 recovery time were independently associated with mean pulmonary arterial pressure and DLCO/VA. The SpO2 reduction time and the 6MWD were not independently associated with mean pulmonary arterial pressure.
Multivariate Linear Regression Analysis Between Outcomes During 6MWT and Clinical Data in Subjects With Chronic Thromboembolic Pulmonary Hypertension
Effects of PEA
The effects of PEA on 6MWT parameters are presented in Table 5. We analyzed changes in 6MWT parameters 1 y post-PEA in 10 of the 17 subjects who underwent PEA, because 7 subjects had measurement error in heart rate and/or SpO2 during the 6MWT. Post-PEA, mean pulmonary arterial pressure, systolic pulmonary arterial pressure, and pulmonary vascular resistance decreased significantly, and Sv̄O2 increased significantly. The 6MWD and heart rate at rest increased post-PEA. Other parameters were unchanged, except for 1 subject whose post-PEA mean pulmonary arterial pressure decreased (from 32 mm Hg to 13 mm Hg), Δheart rate increased (from 43 beats/min to 72 beats/min), slope of heart rate acceleration increased (from 0.3 to 1.1), HRR1 increased (from 25 beats to 48 beats), heart rate acceleration time decreased (from 136 s to 65 s), and SpO2 recovery time decreased (from 123 s to 42 s), in addition to the increase in the 6MWD (from 394 m to 571 m).
Changes in 6MWT Characteristics Observed 1 Y Post PEA in Subjects With Chronic Thromboembolic Pulmonary Hypertension
Discussion
This study has several important findings regarding changes in patterns of heart rate and SpO2 in chronic thromboembolic pulmonary hypertension. Heart rate acceleration was slower, the slope of heart rate was less steep during the 6MWT, and HRR1 was lower after 6MWT in subjects with severe chronic thromboembolic pulmonary hypertension than in those with mild chronic thromboembolic pulmonary hypertension. Additionally, the SpO2 reduction time during the 6MWT and recovery time after the 6MWT were slower in subjects with severe chronic thromboembolic pulmonary hypertension than in those with mild chronic thromboembolic pulmonary hypertension. Importantly, the heart rate acceleration time and slope of heart rate were associated with pulmonary hemodynamics in subjects with chronic thromboembolic pulmonary hypertension.
These results in subjects with severe chronic thromboembolic pulmonary hypertension are consistent with recent studies for PAH, demonstrating small and slow heart-rate change during and after the 6MWT in subjects with PAH.10,17–22 The mechanics of this chronotropic incompetence have been mainly explained as follows: in general, while exercising, PAH subjects exhibit a limited increase in stroke volume,23,24 and the increase in CO is mainly achieved through increases in heart rate. However, chronic overactivity of the sympathetic nervous system leads to downregulation of β-adrenoceptors in the heart,25 which results in a small, slow heart-rate change during exercise in subjects with PAH. Although the physiology of chronic thromboembolic pulmonary hypertension is different from that of PAH (eg, mismatch of ventilation-perfusion, effect of pulmonary dilator), mechanisms similar to those in pulmonary hypertension can be considered in patients with chronic thromboembolic pulmonary hypertension. In patients with chronic thromboembolic pulmonary hypertension, right ventricular afterload increases during exercise, and progression of the disease state eventually causes an impairment in right ventricular function due to chronic obstructions in pulmonary circulation.26 Because the impairment in right ventricular function causes a limited increase in stroke volume during exercise, the heart rate compensates for the demand for increased CO. Our results indicate small and slow heart-rate changes during exercise in subjects with severe chronic thromboembolic pulmonary hypertension. This finding suggests that this limited heart-rate response impairs exercise capacity, possibly suggesting that chronic overactivity of the sympathetic nervous system leads to downregulation of β-adrenoceptors in the heart of chronic thromboembolic pulmonary hypertension patients as well as those with PAH. Because we did not measure circulating catecholamine, we could not draw conclusions regarding these mechanisms in this study. However, a previous study of cardiac I-MIBG uptake indicated left ventricular sympathetic nervous dysfunction in subjects with pulmonary hypertension, including chronic thromboembolic pulmonary hypertension,27 which may support this speculation.
Regarding SpO2 during the 6MWT, SpO2 reduction and recovery time were slower in subjects with severe chronic thromboembolic pulmonary hypertension than in those with mild chronic thromboembolic pulmonary hypertension. However, these parameters were not independently associated with pulmonary hemodynamics. Recovery time of SpO2 associated not only mean pulmonary arterial pressure but also DLCO/VA, while SpO2 reduction time associated CO and LVEF in subjects with chronic thromboembolic pulmonary hypertension. These results suggest that parameters of the change in SpO2 during the 6MWT do not directly reflect hemodynamics. This may be explained at least in part by the complexity of the mechanism of oxygen desaturation during exercise, which has been known to include gas-exchange abnormalities due to an increased dead-space ventilation ratio, a ventilation/perfusion mismatch, and insufficient CO due to right ventricular dysfunction.21,28–30
We have also demonstrated a change in 6MWT parameters both pre- and post-PEA. We originally hypothesized that the heart rate acceleration time would be reduced and the slope of heart rate acceleration would become steep due to improvements in pulmonary hemodynamics after PEA. However, improvement in the 6MWD in this study was only observed among 6MWT parameters, with the exception of 1 subject. Although the explanation for this is unclear, several possible factors can be considered, including reduction of pulmonary hypertension target agents and oxygen due to improved pulmonary hemodynamics, persistence of reduced right ventricular ejection fraction function after PEA,31,32 and small sample size. This issue requires further investigation.
An advantage of our study is that additional components of the 6MWT (ie, heart rate and SpO2 change patterns), which can be easily assessed by clinicians, may have the potential to reveal more precise physiological states of chronic thromboembolic pulmonary hypertension, although right heart catheterization is the gold standard to assess pulmonary hemodynamics in the clinical setting.1,3,33 Echocardiography is also widely used as an initial test for detecting pulmonary hypertension.13 However, the 6MWT is a noninvasive, simple, and inexpensive test that could be repeatedly performed anywhere. Collectively, these results indicate that evaluating heart rate and SpO2 change patterns during the 6MWT is potentially useful for patients with chronic thromboembolic pulmonary hypertension.
Limitations
Our study had the following limitations. This was a single-center retrospective study with a small number of subjects. There are 3 reasons for the small number of subjects: the study required several examinations (eg, right heart catheterization, pulmonary function test, 6MWT, and echocardiography); there were measurement errors in continuous monitoring pulse rate and SpO2 during the 6MWT (Fig. 1); and patients with chronic thromboembolic pulmonary hypertension are relatively rare. Future multi-center studies with larger cohorts of subjects are needed to confirm our results. Another limitation is that we could not conclude the effect of PEA for heart rate and SpO2 change patterns due to multiple confounding factors after the surgery (eg, reduction of pulmonary hypertension target agents and oxygen due to improvement in pulmonary hemodynamics, persistent pulmonary hypertension). In addition, our cohort included many females (27 females vs 4 males in 31 analyzed subjects; 45 females vs 9 males in original 54 patients; see Fig. 1), because chronic thromboembolic pulmonary hypertension is more common in women. However, the results of 4 males (3 mild, 1 severe disease state) show similarities with those of only male subjects (data are not shown). Finally, our study was unable to evaluate the relationship between heart rate patterns during the 6MWT and prognosis, including clinical worsening and mortality. Collectively, further investigation is required.
Conclusions
The change patterns of heart rate and SpO2 during the 6MWT are predominantly associated with pulmonary hemodynamics in subjects with chronic thromboembolic pulmonary hypertension. Therefore, in addition to the 6MWD, evaluating heart rate and SpO2 patterns during the 6MWT might provide a useful, safe, and convenient way to follow the change in pulmonary hemodynamics.
Acknowledgments
We acknowledge Dr Akira Naito, Dr Hajime Kasai, Dr Toshihiko Sugiura, and Dr Rika Suda of the Department of Respirology, Graduate School of Medicine, Chiba University, and Ryogo Ema of the Department of Respirology, Eastern Chiba Medical Center, for their helpful comments.
Footnotes
- Correspondence: Jiro Terada MD PhD, Department of Respirology, Graduate School of Medicine, Chiba University, 1-8-1 Inohana Chuo-ku, Chiba 260-8670, Japan. E-mail: jirotera{at}chiba-u.jp.
Drs Tanabe, Sakao, Terada, and Tatsumi have disclosed relationship with Ministry of Education, Culture, Sports, Science and Technology of Japan, and with The Intractable Respiratory Diseases and Pulmonary Hypertension Research Group, the Ministry of Health, Labor and Welfare, the Pulmonary Hypertension Research Group from the Japan Agency for Medical Research and Development, AMED. Dr Tanabe has received honoraria for lectures from Actelion Pharmaceuticals and Pfizer. Dr Tatsumi has received honoraria for lectures from Actelion. Drs Tanabe and Jujo belong to the Endowed Department, sponsored by Actelion. The other authors have disclosed no conflicts of interest.
- Copyright © 2018 by Daedalus Enterprises