Abstract
BACKGROUND: This meta-analysis aimed to explore the chlorhexidine-related mortality rate for subjects on mechanical ventilation and in an ICU when compared with subjects who received standard ICU care.
METHODS: We searched a number of medical literature databases and the first 100 results in an internet search. Two of us independently reviewed the titles and abstracts of the identified articles. Then general and specific characteristics from eligible articles were extracted and the quality of included trials were appraised by using a risk of bias assessment tool. Risk ratios were calculated, together with the 95% CI. Random-effects models with the Mantel-Haenszel method were used to estimate pooled probabilities. Heterogeneity was identified and quantified via the chi square test and I2 values, respectively.
RESULTS: Eleven of the 547 studies were suitable for this meta-analysis. The included participants were critically ill adults in ICU settings of high-income countries (n = 1157) and low/ middle-income countries (n = 612). They were assigned to either the chlorhexidine or control groups. Overall, moderate-quality evidence indicated reduced ventilator-associated pneumonia incidence (for high-income countries: RR 0.60, 95% CI 0.41–0.87; P = .008; I2 = 39%; and for low- and middle-income countries: RR 0.71, 95% CI 0.51–0.99; P = .05; I2 = 10%), without a substantial effect on mortality rate (for high-income countries: RR 1.01, 95% CI 0.65–1.57; P = .96; I2 = 42%; and for low- and middle-income countries: RR 1.11, 95% CI 0.96–1.29; P = .17; I2 = 0%).
CONCLUSIONS: The prophylactic administration of chlorhexidine among patients who were critically ill and in an ICU setting reduced the occurrence of ventilator-associated pneumonia with no significant impact on associated mortality.
- chlorhexidine
- ventilator-associated pneumonia
- intensive care unit
- evidence-based care
- hospital mortality
- oral rinse
Introduction
Pneumonia is an inflammatory lung condition caused by an overgrowth of microorganisms, including bacteria, viruses, fungi, and parasites, which primarily affect the microscopic air sacs known as alveoli.1,2 Aspiration of bacteria from the oropharynx (ie, upper respiratory tract) into the lower respiratory tract system is the most common mode of infection.1,2 Pneumonia is classified into different types based on the location of acquisition. Ventilator-associated pneumonia (VAP) is a particular type of hospital-acquired (nosocomial) pneumonia that develops after at least 48 h of endotracheal intubation and mechanical ventilation in the ICU.3,4
Three common clinical risk factors of VAP pathogenesis are aspiration, colonization, and impairment of the defense system.2 Other risk factors include an increase of gastric reflux and aspiration probability due to patients' position in bed.1 The incidence of VAP has been reported to be in the range of 8–28%5 and might rise as high as 78%.6 VAP is associated with an extended duration of mechanical ventilation and ICU stay, excessive usage of antibiotics, increased hospital costs, and decreased quality of life.7–9 Furthermore, VAP is also associated with an almost 2-fold increase in mortality compared with similar patients without VAP.10,11
Muscedere et al,12 however, reported a 13.5% mortality rate attributable to VAP in a meta-analysis of 9 randomized controlled trials (RCT). There is increasing evidence in the literature of a strong link between colonization of dental plaque, respiratory pathogens, and VAP.13 Chlorhexidine is an antiseptic plaque growth inhibitor,14 with a number of advantages over other agents, including a low potential for eliciting dermal reactions, good bacteriostatic and bactericidal efficacy, and high intraoral substantivity (ie, sustained antibacterial activity).14,15 Previous systematic reviews reported that chlorhexidine (mouthwash or gel) as part of routine oral hygiene care may decrease VAP occurrence.16–18 Chlorhexidine has been shown to reduce the rate of VAP incidence from 25% to 19% in adults who are critically ill.19
Because of its effectiveness in reducing VAP, chlorhexidine has come into routine practice in critical care units. For example, at the University Health Network in Toronto, basic oral care that includes the use of chlorhexidine is provided for all patients who are intubated and in the ICU.20 However, concerns recently emerged regarding an associated trend toward an increased mortality risk with the use of chlorhexidine. For example, 2 recent meta-analyses reported a trend toward an increased mortality rate with chlorhexidine in an ICU setting with relative risk [RR] of 1.13, 95% CI 0.99–1.2821 and odds ratio of 1.25, 95% CI 1.05–1.50.22 Moreover, a retrospective cohort study at Brigham and Women's Hospital, Boston, Massachusetts reported the similar trend with a hazard ratio [HR] 1.63, 95% CI 1.15–2.31.23 In contrast, a recent hospitalwide retrospective cohort study (at Ghent University Hospital, Ghent, Belgium) included a large sample of 82,274 subjects, of whom 11,133 received chlorhexidine oral care.24 They identified no harmful effects in the subjects on ventilation and in those not on ventilation while in the ICU, and recommended against indiscriminate widespread use of chlorhexidine in hospitals. Based on these new findings, some guidelines for the management of VAP have either moved away from recommending chlorhexidine or are pending their recommendations until more safety data become available.25
In light of further analysis of the best available evidence regarding the efficacy of chlorhexidine on VAP prophylaxis and its association with ICU, the mortality rate is required. This review aimed to evaluate the impact of prophylactic chlorhexidine administration on the mortality rate and VAP incidence in patients in the ICU of high-income countries23 because their health-care systems are similar to that of Canada. In particular, this article aimed to summarize the best available evidence for each outcome, mortality, and VAP incidence, and to discuss if it would be worthwhile continuing to use chlorhexidine in the ICU.
Methods
To identify studies for this review, we developed a detailed search strategy similar to a previous systematic review16 on this topic by adhering to Preferred Reporting Items for Systematic Reviews and Meta-Analysis criteria.26 We focused our search on adult populations in high-income countries. The following population, intervention, control, and outcome eligibility criteria were developed:
Population: Our primary population of interest was ventilated adult subjects in ICU settings of high-income countries (ie, gross national income per capita ≥ $12,23627). We also included studies of adult subjects on ventilation and in ICU settings of low- and middle-income countries for separate analysis. Other inclusion criteria were no previous intubation, no baseline clinical pneumonia, and a need of mechanical ventilation for at least 48 h. We excluded studies on pediatric subjects.
Intervention: Our intervention was standard ICU care, with chlorhexidine application of multiple concentrations at 0.12% and 0.2% in gel and solution modes of delivery (excluding antibiotic use) as a preventive therapy for VAP in the ICU. The antibiotic therapies excluded from the studies refer to selective decontamination by using topical antibiotics.
Control: For the control group, we designated placebo or standard ICU care without chlorhexidine application as a preventive therapy for VAP in the ICU.
Outcomes: We included the studies in which mortality (defined as ICU mortality (directly and indirectly attributable) and VAP incidence (defined as pneumonia that developed after at least 48 h of endotracheal intubation and mechanical ventilation in the ICU) were reported.
Search Methods for Identification of Studies
Our search strategy is highlighted in Appendix 1 (see the supplementary materials at http://www.rcjournal.com). We searched CENTRAL (issue 9 of 12, September 2018), MEDLINE (Ovid) (1946 to September week 4, 2018), and Embase (Ovid) (1947 to September week 4, 2018). To obtain unpublished related information, we searched the first 100 results in Google and contacted the authors of the included studies. We further hand-searched bibliographies of the eligible RCTs. No language restriction was applied.
Data Collection
Two of us (SL and NLL) independently reviewed the title and abstract of identified results and extracted general and specific trial characteristics related to our population, intervention, control, outcome eligibility criteria question from eligible articles by using a predesigned standard data collection form. Extracted general characteristics of the study included authors, publication year, and the country where the study was conducted. Characteristics of the study design required for the risk of bias appraisal were also included. Characteristics related to subjects included the reason for their hospitalization and their age. Details of the interventions and controls, such as dosage and mode of delivery, were recorded. Outcomes and their timings were also recorded, as defined above. We resolved disagreements through discussion and consensus with another one of us (AA). Authors were contacted for further clarification if necessary.
Assessment of Risk of Bias in Included Studies
Two of us (SL and NLL) independently evaluated the quality of included trials by using the Cochrane “risk of bias” assessment tool.28 We evaluated 6 domains (ie, sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other issues) based on what was reported in each study. We assigned a judgment (such as “high risk,” “low risk,” or “unclear risk” of bias) that related to the risk of bias for that particular domain.
Quantitative Data Synthesis
In the meta-analysis, we analyzed summary effects by considering RR, together with the 95% CI calculated for dichotomous outcomes by using Review Manager 5.2.7 software (Cochrane Collaboration, Oxford, United Kingdom). Random-effects models, with the Mantel-Haenszel method, were used to estimate pooled probabilities within the chlorhexidine and control populations. Our primary meta-analysis focused on studies of adult subjects on ventilation and in ICU settings of high-income countries because of their comparability with the Canadian health-care system. We also reported a supplemental analysis of the adult subjects on ventilation and in ICU settings of low- and middle-income countries. With assuming that significant differences may exist between the ICU settings of high-income compared with low- and middle-income countries, we elected not to pool these studies. We inspected the graphic display of the trials' estimated treatment effects (along with their 95% CIs) to assess heterogeneity in the generated forest plots. To identify and quantify heterogeneity, we used a chi square test (with a significance level of 0.1 as the cutoff value) and I2 statistic values, respectively.29 I2 values were used based on suggestions from the Cochrane Handbook for Systematic Reviews of Interventions.30
Subgroup and Sensitivity Analyses
Potential heterogeneity among studies was explored in a number of a priori subgroup and sensitivity analyses. We stratified data into subgroups based on the location of the study (studies from high-income countries vs studies from low- and middle-income countries) as well as chlorhexidine concentrations and mode of delivery. We also performed sensitivity analysis to explore the risk of bias on outcomes, excluding studies with unclear diagnostic criteria for VAP or with a high risk of bias, or of changing the assumptions of attrition biased data (ie, missing data) when calculating the effectiveness.
Results
Description of Included Studies
The study flow diagram is presented in Figure 1. After removing duplicates, the electronic search strategies identified 547 articles. Of these, 42 studies were selected for full-text review and 11 of these 42 studies were included for this review. The characteristics of these 11 included studies are explained in Table 1. Reasons for excluding 31 studies are provided in Appendix 2 (see the supplementary materials at http://www.rcjournal.com). Six RCTs were conducted in the hospital ICU setting of high-income countries (3 in the United States,31–33 2 in France,34,35 and 1 in Australia36). Another 5 RCTs were conducted in the hospital ICU setting of low- and middle-income countries: 1 study from each of the following: Brazil,37 Turkey,38 Croatia,39 Thailand,40 and India.41 The assigned risk of bias and the methodological aspects for each included study are shown in Figure 2. Overall, 2 studies34,37 were appraised at “low risk of bias” for all domains. Nine studies31,32,35,36,38–41 were appraised at “unclear risk of bias” for at least one domain, and 3 studies31,36,40 were appraised at “high risk of bias” for at least one domain.
Flow chart. VAP = ventilator-associated pneumonia.
A: Summary of risk of bias. B: Our judgments about each risk of bias domain presented as percentages across all included studies.
Characteristics of Eligible Studies
Participants
A total of 1,157 adult participants who were critically ill with a range of medical and surgical (including trauma) conditions were included in the 6 RCTs from high-income countries31–36, and 612 subjects were included from 5 RCTs for the low- and middle-income countries37–41. The details are listed in Table 1.
Classification of the Interventions
Nine included studies had one experimental arm of gel or solution chlorhexidine,33–41 one study had 2 experimental arms of placebo plus chlorhexidine and chlorhexidine alone,31 and the last study had 3 experimental arms of chlorhexidine, tooth brushing, and chlorhexidine plus tooth brushing32 (Table 1). The following 3 groups of interventions were used singly or in combinations with other treatments, as mentioned above (eg, tooth brushing, sterile water): chlorhexidine solution (0.12%,31–37 2%,40 0.2%36,38,41), and chlorhexidine gel (0.2%34,35,39).
Classification of the Controls
The following groups of controls were used singly or in combinations: placebo or usual care without chlorhexidine,33,34,37,39 water,36 saline solution,38,40 0.01% potassium permanganate,41 and bicarbonate isotonic serum.35
Effects of Interventions on Mortality Incidence
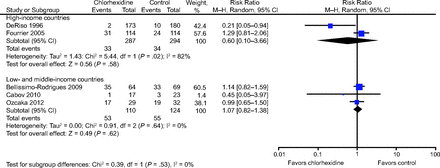
Results from our meta-analysis suggested that there was no evidence of a significant effect of chlorhexidine on ICU mortality (for high-income countries: RR 1.01, 95% CI 0.65–1.57; P = .96; I2 = 42%; and for low- and middle-income countries: RR 1.11, 95% CI 0.96–1.29; P = .17; I2 = 0%) (Fig. 3).
Forest plot, comparing chlorhexidine vs control for mortality. Subgroup analysis was based on income.
Effects of Interventions on VAP Incidence
Results from our meta-analysis showed a statistical difference between chlorhexidine and control groups in reducing the VAP incidence (for high-income countries: RR 0.60, 95% CI 0.41–0.87; P = .008; I2 = 39%; and for low- and middle-income countries: RR 0.71, 95% CI 0.51–0.99, P = .05; I2 = 10%) (Fig. 4).
Forest plot, comparing chlorhexidine vs control for ventilator-associated pneumonia incidence. Subgroup analysis was based on income.
Sensitivity Analysis
Our sensitivity analysis (Fig. 5) focused on studies with a low risk of bias and stronger methodological quality and found no difference in the mortality incidence for high-income countries (2 trials33,34: pooled RR 0.60, 95% CI 0.10–3.66; P = .58; I2 = 82%) as well as for low- and middle-income countries (3 trials37–39: pooled RR 1.07, 95% CI 0.82–1.38; P = .62; I2 = 0%). Similarly, no difference in the VAP incidence was identified for both settings (high-income countries: RR 0.60, 95% CI 0.17–2.09; P = .42; I2 = 76%; and low- and middle-income countries: RR 0.71, 95% CI 0.42–1.19; P = .19; I2 = 35%) (Fig. 6).
Forest plot, comparing chlorhexidine vs control for mortality. Sensitivity analysis.
Forest plot, comparing chlorhexidine vs control for ventilator-associated pneumonia incidence. Sensitivity analysis.
Subgroup Analysis
Our subgroup analyses based on chlorhexidine concentrations (0.12%, 0.2%, and 2%) and mode of delivery (gel or rinse) showed no difference in the mortality incidence (Fig. 7 and Table 2). With regard to the VAP incidence, our subgroup analysis showed that a chlorhexidine solution and chlorhexidine 0.2% significantly reduced the VAP incidence (Fig. 8 and Table 2); however, chlorhexidine gel, chlorhexidine 0.12%, and chlorhexidine 2% only slightly (but not significantly) reduced the VAP incidence rate.
Forest plot, comparing mode of delivery and chlorhexidine concentration effectiveness on mortality.
Forest plot, comparing mode of delivery and chlorhexidine concentration effectiveness on ventilator-associated pneumonia incidence.
Summary of Main, Subgroup, and Sensitivity Analyses for the Outcomes of Incidences of Mortality and Ventilator-Associated Pneumonia
Discussion
Our meta-analysis identified 11 RCTs that would answer our a priori defined the population, intervention, control, outcome eligibility criteria question. To better relate our results to the Canadian health-care system, we decided to perform 2 separate analyses based on studies from high- versus low-and middle-income countries. The pooled evidence from these included trials showed a reduction of VAP incidence of ∼40% and 30% in the chlorhexidine groups of high-income countries and low-and middle-income countries, respectively. This finding was in agreement with previous meta-analyses that reported that chlorhexidine (mouthwash or gel) as part of routine oral hygiene care can reduce VAP occurrence16–19,42 (eg, RR 0.72, 95% CI 0.55–0.94; P = .02 as reported in Labeau et al42; and RR 0.75, 95% CI 0.62–0.91; P = .004 among adults who were critically ill, as reported in Hua et al19).
Our analysis identified no difference in the mortality incidence between chlorhexidine and control groups. However, the pooled estimates are imprecise (Fig. 3), and the results are open to controversy when compared with other studies. For example, similar to our finding, a 2013 Cochrane systematic review16 concluded no evidence of a difference between chlorhexidine and control in the outcomes of mortality (odds ratio 1.10, 95% CI 0.87–1.38). However, Price et al21 suggested that chlorhexidine may increase mortality (odds ratio 1.25, 95% CI 1.05–1.50). The main difference in the outcome between these meta-analyses pertains to differences in the studies included and the interpretation of each study. It is unclear why chlorhexidine can increase mortality rates.
Chlorhexidine is known to be highly potent and has a broad bactericidal spectrum. It acts rapidly on microbes and destabilizes the negatively charged sites on their cell wall.15 Therefore, it is possible that a routine application of chlorhexidine may result in antibacterial resistance and modification of oral microbial flora that may result in an adverse effect.15,43 That said, such an impact during the relatively short administration time in the ICU is unexpected. Chlorhexidine may also have a prolonged activity in patients in the ICU. It is reported that antimicrobial activity of chlorhexidine can last at least 48 h on the skin and in the oral cavity.44 Chlorhexidine has an intraoral substantivity (ie, sustained antibacterial activity) because it binds to oral tissue such as oral mucosa and teeth, and is released slowly.45,46 When chlorhexidine oral rinse is provided to patients who are critically ill, it may not be completely rinsed out by nurses. When coupled with its substantivity, which allows for a long duration of antimicrobial action, the remainder that did not get suctioned out may result in toxic effects and, hence, an increased risk of mortality.
Many factors interact with each other and can have significant effects on the overall ICU outcome. Some of these factors include the health-care provider to patient ratio, level of medical intensive care response team, presence of an intensivist, and available technological and pharmacologic facilities. Furthermore, ICU performance may be compared by different measures, such as VAP and central line infection rate, 48-h readmission rate, and nurses with critical care training.47 Because we were not able to compare individual factors from each ICU setting that were included in our study, we assumed that significant differences may exist between the ICU settings of high income compared with low- and middle-income countries, and, hence, we investigated these settings separately without pooling the results. Overall, the results from ICU setting of low-and middle-income countries were similar to those of studies from high-income countries. However, in comparison with high-income countries, there was a slight trend of overall higher mortality and VAP incidence rates in studies from low- and middle-income countries.
Our meta-analysis showed a decrease in the VAP incidence rate in chlorhexidine groups, with no statistically significant change in the mortality rate between the treatment and control groups. These results were obtained despite some limitations, such as incompleteness of reports in studies and the potential for a high or unclear risk of bias. Our meta-analysis supported the prophylactic administration of chlorhexidine among patients who were critically ill. Future research and their appropriate reports can shed more light on this matter, in particular, by adhering to the use of the Consolidated Standards of Reporting Trials (CONSORT) statement when reporting RCT results and reporting necessary details of each domain in methodology, enabling blinding, if possible, through indistinguishable placebo treatment, detailed reports of the VAP diagnosis method, and interventions and their adverse effects, if any.
Conclusions
There was a moderate amount of quality evidence that showed that the prophylactic administration of chlorhexidine among patients who were critically ill and in an ICU setting reduced the occurrence of VAP, with no significant impact on associated mortality.
Footnotes
- Correspondence: Amir Azarpazhooh DDS PhD, Faculty of Dentistry, University of Toronto, 455 – 124 Edward Street, Toronto, ON, M5G 1G6, Canada. E-mail: amir.azarpazhooh{at}utoronto.ca.
The authors have disclosed no conflicts of interest.
Supplementary material related to this paper is available at http://www.rcjournal.com.
- Copyright © 2019 by Daedalus Enterprises