Abstract
BACKGROUND: The present study aimed to characterize the behavior of 3 components of respiratory muscle function during mechanical ventilation weaning in children to better understand the respective impact of a spontaneous breathing trial on ventilatory mechanical action (esophageal pressure [Pes], ventilatory demand (electrical activity of the diaphragm [EAdi]), and oxygen consumption.
METHODS: This was a prospective single-center study. All children > 1 months and <18 y old who were intubated and on mechanical ventilation, and who were hospitalized in the pediatric ICU were eligible. Subjects considered as ready to extubate were included. Simultaneous recordings of oxygen consumption, Pes, and EAdi were performed during 3 steps: before, during, and after the spontaneous breathing test.
RESULTS: Twenty subjects (median age, 5.5 mo) were included. Half of them were admitted for a respiratory cause. The increase in Pes swings and esophageal pressure-time product during the spontaneous breathing trial was not significant (P = .33 and P = .75, respectively), and a similar trend was observed with peak EAdi (P = .06). Oxygen consumption obtained by indirect calorimetry was stable in the 3 conditions (P = .98).
CONCLUSIONS: In these children who were critically ill, a spontaneous breathing trial induced a moderate and nonsignificant increase in work of breathing, as reflected by the respiratory drive with EAdi and respiratory mechanics with Pes. However, indirect calorimetry did not seem to be a sensitive tool to assess respiratory muscle function during the weaning phase in children who were on mechanical ventilation, especially when work of breathing was slightly increased.
Introduction
Respiratory failure is the leading cause of admissions in the pediatric ICU and mechanical ventilation is first-line treatment. Although mechanical ventilation is often lifesaving, it can be associated with complications, such as ventilator-induced lung injury,1 nosocomial infection, airway trauma, and severe diaphragmatic dysfunction.2 It, therefore, is important that mechanical ventilation be discontinued as soon as possible, that is, as soon as the patient is capable of spontaneous breathing.
During the weaning phase of mechanical ventilation, the ability of the respiratory muscles to cope with the imposed load is challenged.3 The assessment of respiratory muscle function during the weaning process could help to predict extubation success and optimize its timing.4 Different tools are available to monitor different components of respiratory muscle function during the weaning phase. Esophageal pressure (Pes) measurement is the reference method to measure the pressure generated by the patient's inspiratory muscles and has been shown to be a good method to predict weaning outcome.5,6
The electrical activity of the diaphragm (EAdi) reflects the patient's ventilatory drive, and it has been used to monitor diaphragm activity during weaning trials.7 The neuromechanical or neuroventilatory efficiency of the respiratory system can be estimated by the ratio of the muscle pressure or the ventilatory volume divided by the corresponding EAdi.8 Moreover, oxygen consumption (V̇O2) has been shown to change on adaptations of mechanical ventilation parameters and modes because the oxygen cost of breathing reflects the output of the respiratory muscles.9
Whereas, in normal subjects, the portion of V̇O2 assigned to the respiratory load V̇O2 accounts for 1 to 3% of the whole body V̇O2,10 this percentage can be considerably higher in patients being weaned from ventilation.11,12 Indirect calorimetry, which provides V̇O2 continuously at the bedside, could be an attractive tool to assess the respiratory muscle function because this could be a reliable and less invasive method of estimating the change in the work of breathing. However, pediatric data in this field are lacking, especially about the reliability and reproducibility of indirect calorimetry. The present study aimed to characterize the behavior of 3 components of respiratory muscle function during mechanical ventilation weaning in children to better understand the respective impact of a spontaneous breathing trial (SBT) on Pes, EAdi, and V̇O2.
QUICK LOOK
Current Knowledge
The assessment of respiratory muscle function during the weaning process could help to predict extubation success and optimize its timing. Different parameters (such as esophageal pressure, electrical activity of the diaphragm, and oxygen consumption measured by indirect calorimetry) are available to monitor various components of respiratory muscle function during the weaning phase.
What This Paper Contributes to Our Knowledge
In children who were critically ill, a spontaneous breathing trial induced a moderate and nonsignificant increase in work of breathing, as reflected by the respiratory drive monitored with electrical activity of the diaphragm and by the respiratory mechanics measured with esophageal pressure. However, indirect calorimetry, which measures oxygen consumption, did not seem to be a sensitive tool to assess respiratory muscle function during the weaning phase in children on mechanical ventilation.
Methods
This was a prospective study, conducted in the pediatric ICU of Centre Hospitalier Universitaire (CHU) Sainte-Justine, a university affiliated pediatric hospital, from November 2016 to June 2017. The study protocol was approved by the ethics committee of CHU Sainte-Justine, and written informed consent was obtained from the parents or legal tutor.
Subjects
Consecutive children ages between 1 month and 18 y admitted to the pediatric ICU, intubated, and on mechanical ventilation were eligible. The screening was performed by the research assistants Mariana Dumitrascu, Mary-Ellen French, and Jouher Nait every working day. Eligible patients were included if they were deemed to be ready for an extubation readiness test as per the attending team, provided the research team and the equipment were available. Patients were excluded if they had one of the following criteria: contraindications to the placement of a new nasogastric tube, hemodynamic instability, severe respiratory instability, axillary temperature > 38°C, cuff leaks > 10%, nitrite oxide therapy, ventilation with high frequency oscillation, or if a limitation of life support treatments was discussed or decided. Detailed inclusion and exclusion criteria are given in the supplementary material (see the supplementary materials at http://www.rcjournal.com).
Protocol and Data Recording
Pes, EAdi, and V̇O2 data were monitored to characterize respiratory muscle function. Pes and EAdi were obtained simultaneously by a specific modified 8 French EAdi catheter (NeuroVent Research, Toronto, Ontario, Canada) equipped with both microelectrodes (for EAdi monitoring) and an esophageal balloon (for Pes monitoring) (see the supplementary materials at http://www.rcjournal.com), installed 30 min before measurement. The catheter was connected to a Servo-i ventilator (Maquet, Solna, Sweden), to gather the EAdi signal. The correct position of the catheter was checked with a specific positioning screen on the Servo-i by following the company recommendation.13 In that position, the middle electrodes of the array are very close to the median diaphragm position. The balloon, which is located 1 cm above the upper electrode, therefore, was in the lower esophagus. Pes and EAdi signals were simultaneously recorded by using an acquisition system (NeuroVent Monitor XIII) and displayed and analyzed by using specific software (NeuroVent Research).
V̇O2 and resting energy expenditure were measured by a portable indirect calorimeter (Vmax Encore, CareFusion, Yorba Linda, California), which was connected 30 min before measurement. Gas and pressure were calibrated according to the manufacturer's instructions. All data were measured during 3 periods of 30 min each: before, during, and after an SBT in CPAP. The subjects were supine, with the head of bed elevated to 30°, and no or light sedation, according to the attending physician's instructions.
Demographic data and subjects characteristics, including age, sex, weight, time of measurements, admission diagnostic, and comorbidities, and Pediatric Risk of Mortality and Pediatric Logistic Organ Dysfunction scores were collected. Rapid shallow breathing index is defined as the ratio of breathing frequency to tidal volume (VT). Predicted energy expenditure was calculated according to the Schofield equations (kcal/d) (see the supplementary materials at http://www.rcjournal.com). Clinical indices of increased work of breathing and comfort of the subject, assessed with the COMFORT behavior scale, were gathered during the 3 periods. The sedation level was assessed with the sedation score described by Randolph et al,14 which was adapted to include dexmedetomidine according to the study performed by Eren et al.15 One point was given for morphine or midazolam equivalents of 0.1 mg/kg. Pediatric ICU outcome data included the following: SBT success or failure, extubation success or failure (as defined by the need to re-intubate in the 48 h after extubation), and duration of mechanical ventilation.
Data Analysis
For Pes and EAdi recordings, after elimination of the cycles with artifacts, such as coughing or esophageal spasms, at least 20 consecutive breath cycles in the 3 different respiratory conditions were used for subsequent analysis. Pes–time product (PTPes) was obtained by measuring the area under the Pes signal between the onset of inspiration and the end of inspiration as determined on EAdi tracing. PTPes was also expressed per minute by multiplying the PTPs per breath by the breathing frequency (PTPes/min). Neuromechanical and neuroventilatory efficiencies were assessed by the ratio of swing Pes to peak EAdi, and VT to peak EAdi, respectively.
The mean V̇O2 and resting energy expenditure were calculated during the 3 periods. Data from the first 10 min and from periods of documented physical movement or nursing care, such as endotracheal suctioning, were excluded. The steady state was defined as 10 consecutive minutes during which V̇O2 and carbon dioxide production vary less than ±10%, as previously described.16 The respiratory load V̇O2 was defined as the difference between V̇O2 during SBT and V̇O2 under conventional mechanical ventilation.
Statistical Analysis
Data were expressed as median values (interquartile range [IQR]) for continuous variables and as number and/or frequency (%) for categorical data. Differences for categorical variables were tested by using the chi-square or the Fisher exact test (when expected frequencies were < 5). Differences in continuous variables were assessed by using the non-parametric Mann-Whitney test or the paired t test, depending on the variable distribution. Repeated measures analysis of variance was used to compare the different conditions (before, during, and after SBT in CPAP) on the different variables. Pearson correlation coefficient (R) and determination coefficient (R2) were used to evaluate the correlation between changes in swing Pes, PTPes, EAdi, and V̇O2. All P values were 2-tailed and considered significant if P < .05. Statistical analyses were performed by using SPSS 24.0 (SPSS, Chicago, Illinois). Details regarding the sample size are given in the supplementary material (see the supplementary materials at http://www.rcjournal.com).
Results
Study Population
During the study period, 568 patients were admitted to the pediatric ICU, and 280 were mechanically ventilated. Thirty-one patients were eligible and approached by the study team. Five parents or guardians did not consent to the study, whereas 4 patients were extubated before inclusion. In 2 subjects, measurements were not performed due to clinical deterioration. In total, 20 subjects were enrolled (10 boys), with a median age of 5.5 (IQR, 2–64) months. The subjects' characteristics are presented in Table 1. They were studied 7 (IQR, 3-11) d after initiation of mechanical ventilation. The main reason for admission to the pediatric ICU was respiratory failure (n = 10 [50%]), and 3 subjects (15%) were admitted after surgery. Before SBT, the median COMFORT score was 14 (IQR, 12-16). Five subjects (25%) received intermittent analgesic treatments only, whereas 5 (25%) received both sedative and analgesics agents with a continuous infusion. In 2 subjects, EAdi could not be measured due to technical problems and, for another subject, the measurements from indirect calorimetry were inaccurate due to air leaks of >10% that occurred after inclusion. All the subjects were on the same settings after SBT as before SBT.
Characteristics of the Study Population
Breathing Pattern and Work of Breathing During SBT
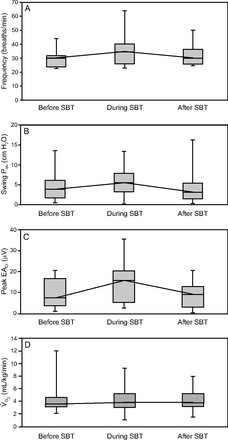
As detailed in the Table 2, the breathing frequency tended to increase during the SBT, although this did not reach significance (P = .23). No clear changes in VT, minute ventilation, and the rapid shallow breathing index were observed. Unexpectedly, the increase in Pes swings and PTPes/min during the SBT was not significant (P = .33 and P = .75, respectively) (Fig. 1). Pes swings increased by at least 10% during SBT in 14 subjects (70%). A similar trend was observed with peak EAdi (P = .059). Neuromechanical and neuroventilatory efficiency did not change significantly before, during, or after SBT. We did not observe any association between Pes and EAdi changes and the level of comfort or the sedation score.
Clinical Status and Respiratory Muscle Assessment Before, During, and After SBT
Work of breathing before, during and after SBT, assessed by breathing frequency (A), swing peak esophageal pressure (Pes, B), peak electrical activity of the diaphragm (EAdi, C), and oxygen consumption (V̇O2, D). SBT = spontaneous breathing trial.
Variables Measured By Indirect Calorimeter
V̇O2 obtained by indirect calorimetry was stable in the 3 conditions (P = .98). In 10 subjects (50%), V̇O2 decreased during SBT. Resting energy expenditure and the respiratory load V̇O2 patterns did not differ according to the COMFORT and sedation score. Predicted resting energy expenditure was overestimated compared with the measured resting energy expenditure (47 [40-54] kcal/kg/d vs 23 [21-29] kcal/kg/d, P < .001) and the median (IQR) respiratory quotient measured by indirect calorimetry was 0.71 (0.69–0.78).
Correlations Between Pes, EAdi, and V̇O2
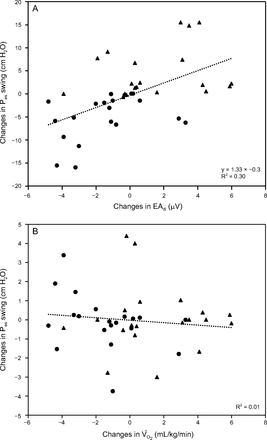
Overall, changes in V̇O2 were not correlated with the changes observed with PTPes and peak EAdi (R2 = 0.05 and R2 = 0.01, respectively). Similarly, only a poor correlation was observed between changes in Pes and changes in EAdi (R2 = 0.3) (Fig. 2).
Relationship between the changes of swing ventilatory mechanical action (esophageal pressure [Pes]) vs peak ventilatory demand (electrical activity of the diaphragm [EAdi]) (A) and swing Pes vs oxygen consumption (V̇O2) (B) from conventional settings to spontaneous breathing trial (SBT) (▴) and from SBT to previous settings (●).
Outcome
SBT was considered a success in 20 subjects (100%), and extubation was successful in 17 subjects (89%). One subject was not extubated and died after limitation of life support treatment. Median (IQR) duration of mechanical ventilation and stay in the pediatric ICU were 10 (4-14) d and 18 (7-42) d, respectively. After extubation, 9 subjects (45%) required no respiratory support, whereas 2 (10%) were reintubated, and 8 (40%) were on noninvasive respiratory support within 1 h of extubation. The pattern of PTPes, EAdi, and V̇O2 during the SBT were not associated with extubation failure, although the number of subjects was limited.
Discussion
In this series of 20 children who were critically ill, we observed that SBT in CPAP was associated with a nonsignificant increase in Pes derived indices of effort of breathing and of EAdi. However, the respiratory load V̇O2 measurements were stable during the maneuver. In children, the benefit of weaning protocols, including SBT, to shorten the duration of ventilation has been demonstrated,17 and the recent consensus conference on pediatric ARDS recommends that SBT and/or extubation readiness tests should be performed.18 For monitoring patient-ventilator interactions during SBT, clinicians mostly rely on patient breathing patterns and waveforms that are available on most ventilators.
However, several difficulties remain to assess the patient's contribution to the work of breathing with usual clinical monitoring during mechanical ventilation.19 Measurements of Pes and EAdi provide the clinicians with a bedside evaluation of respiratory effort from 2 different points of view. Assessment of Pes is the reference method to measure the pressure generated by the patient's inspiratory muscles. The direct monitoring of respiratory muscle function by Pes has been shown to be helpful in titrating ventilator support in different pathologies20 and, as Jubran and Tobin5 showed in adults, the Pes trend during an SBT can provide an accurate prediction of weaning outcome.
However, EAdi gives the clinician a bedside evaluation of diaphragm activity.21 EAdi can be considered as a parameter that reflects patient ventilatory drive, and it provides a continuous indirect reflection of the evolution in work of breathing.22 Because adequate diaphragmatic function is paramount for weaning success, the relevance of monitoring EAdi during the weaning process has also been described.23 Wolf et al7 observed that the ability to generate a higher diaphragmatic activity for the same VT in pressure-support ventilation was a predictor of successful extubation. Combining EAdi and VT in the neuroventilatory index is an interesting concept because the comparison of ventilatory demand with mechanical output provides information on diaphragmatic efficiency.
Our initial hypothesis was that V̇O2 provided at the bedside of children who are critically ill by indirect calorimetry could provide a reliable and less-invasive method to estimate the change in the work of breathing during weaning from conventional ventilation. We reasonably assumed that no confounding factor except increasing the work of breathing could induce an increase in V̇O2 during SBT. We, therefore, chose to record 3 periods of 30 min, during which time, the underlying metabolic condition could not change, as suggested in a previous study.12
Many investigators, rather than focusing on mechanical muscle function, evaluated the role of the respiratory load V̇O2 as a predictor of weaning success,9,24 and other investigators reported that the respiratory load V̇O2 was closely correlated to PTP.25 In children, the use of indirect calorimetry for respiratory purposes is scarcely reported.26 In a few studies, V̇O2 has been measured in children on mechanical ventilation in different clinical conditions to assess the effects of therapeutics such as neuromuscular blockers27 or inhaled salbutamol.28 To our knowledge, V̇O2 measurement during weaning from pediatric mechanical ventilation has never been studied.
Compared with measurements from Pes and EAdi, the respiratory load V̇O2 did not tend to accurately reflect changes in work of breathing. We could expect a respiratory load V̇O2 of ∼10% of V̇O2 according to the study by Hoher et al29 who found that subjects on assisted ventilation spent 11% more energy than those on controlled ventilation, as supported by another study.12 Yet, we found, in the present study, that the respiratory load V̇O2 was not influenced by ventilator mode, in agreement with the study published by Briassoulis et al30 in a population of 11 children who were critically ill and on mechanical ventilation.
We suggested several hypotheses to analyze this finding. First, the subjects were relatively stable and had a low level of support before SBT. In that context, the respiratory load V̇O2 may not be as sensitive as Pes or EAdi to detect a small change in the work of breathing. According to the study performed by Frankenfield and Ashcraft,31 measurements from the Vmax Encore Calorimeter can be biased by 5% in the same subject. One may speculate that, in patients with worse respiratory function, the results might have been different. Second, this finding could be explained by the great heterogeneity of our population, in terms of pathologies, mode of ventilation, ventilator settings, duration of ventilation, time of inclusion, and degree of sedation.30 Third, SBT may induce changes in functional residual capacity, which results in changes in spirometer volume unassociated with V̇O2.
Also, it is important to note that agitation or distress that may accompany a difficult SBT can also contribute to the V̇O2 increase. In our study, the changes in V̇O2 were small, and similar values of COMFORT behavior scores were observed during the 3 periods of the protocol (before, during, and after SBT), which made it difficult to assess this potential relationship. Further work is clearly needed to assess the value of indirect calorimetry during the weaning phase in other contexts and, in particular, earlier in the course of ventilation and in patients with a worse respiratory condition and a higher rate of SBT failure.
Besides the main study goal, we, interestingly, observed absolute V̇O2 values that were lower than previously reported.26,32 Most of these studies were performed at the early stage of the disease.33 Moreover, most of the subjects in those studies were admitted after trauma, and some of them received nutritional therapy, which could explain such difference. In our study, because we performed indirect calorimetry measurements during the weaning phase, we hypothesized that the metabolic rate was low in the recovery period. Although we consider observed V̇O2 values as true, we cannot exclude that confounding factors could explain such results. First, although we excluded patients with FIO2 > 0.6 or cuff leaks > 10%, values could be less accurate in subjects with FIO2 > 0.4 or cuff leaks > 5%.34 In addition, even though we used a standardized and validated approach for indirect calorimetry measurements, we could not rule out the possibility of inaccurate measurements or technical problems.35
Both EAdi and Pes are well-known parameters that indirectly reflect respiratory muscle function. Previous studies show a tight correlation between EAdi and Pes.22,36 However, there is high inter-individual variability.36 In our study, the changes observed between the 3 periods for these 2 variables were relatively small, and the data were limited, which likely explained the poor correlation observed, due to limited statistical power. This can also reveal that the effort needed to breathe before SBT was not significantly increased over normal physiologic conditions, which would not have been the case if measurements had been performed earlier in the course of mechanical ventilation.
Of note, the relationship between EAdi and Pes can also be affected by sedation37 or by the level of assistance.38 These factors, in addition to the great heterogeneity in our population, could also explain the moderate correlation between EAdi and PTP. An impaired neuromuscular coupling39 seemed unlikely in these subjects with a near-normal respiratory condition. Also, our findings regarding the difference between predicted and measured resting energy expenditure were in agreement with those reported elsewhere. Indeed, several studies showed that predictive equations overestimated resting energy expenditure in children who were critically ill and with various clinical situations.32,40 Our results tended to confirm that such equations could not substitute for indirect calorimetry measurement of resting energy expenditure.
There were several limitations in the present study. This was a single-center study, which tends to limit its generalizability. The sample size was small and, therefore, this study may have been underpowered to demonstrate the correlation between the variables and to show any significant impact of SBT on the indices that reflected the effort of breathing. The sample size had been estimated when we hypothesized a greater impact of SBT and a high correlation between the 3 methods of work of breathing assessment. We performed measurements during daytime and weekdays only, which could lead to a selection bias. The study population was heterogeneous in terms of reasons for admission, duration of mechanical ventilation, age, and degree of sedation of the patients. This may lead to different respiratory behavior during weaning from mechanical ventilation. However, the population reflected a usual pediatric ICU population.
In addition, work of breathing indices were assessed during a 30-min SBT, whereas some investigators have suggested a longer extubation readiness test.20 Nevertheless, significant changes in respiratory mechanics occur rapidly after modification of ventilator settings. Regarding the Pes measurement, due to the unique catheter used to monitor both EAdi and Pes, it was not possible to independently adjust the position specifically for Pes, although the position in the lower esophagus was relatively guaranteed by the probe design. It was possible that this induced a suboptimal Pes signal in some subjects. Noteworthy, the impact of the position was likely the same during the 3 ventilation periods. Finally, we did not assess respiratory muscle function after extubation.
Conclusions
In these children who were critically ill, an SBT on CPAP induced a moderate and nonsignificant increase in the work of breathing, as reflected by the respiratory drive with EAdi and by respiratory mechanics with Pes. In this specific population with relatively stable respiratory status, the V̇O2 measurement was not a sensitive parameter to detect an increase in respiratory muscle activity during the SBT. The interest of this monitoring technology should be further evaluated, in particular, in patients with a higher work of breathing.
Acknowledgments
The authors thank the patients and their families for their willingness to participate in our study. We thank Mariana Dumitrascu, Mary-Ellen French, and Jouher Nait for their help in the screening and enrollment process, Lucy Clayton for the study management support, the respiratory therapists for their logistic help, the pediatric ICU fellows, attending health-care providers, and pediatric ICU nurses for their collaboration.
Footnotes
- Correspondence: Guillaume Mortamet MD PhD, Pediatric Intensive Care Unit, CHU Sainte-Justine, 3175 Côte Sainte-Catherine, Montreal, Quebec H3T 1C5, Canada. E-mail: mortam{at}hotmail.fr.
The study was supported by the Réseau en Santé Respiratoire du Québec, the Assistance Publique des Hôpitaux de Paris, and the Fondation Sainte-Justine.
This work was performed in CHU Sainte-Justine, Pediatric Intensive Care Unit, Montreal, Quebec, Canada.
Dr Mortamet presented this work at the National Congress on Intensive Care Medicine, held on January 23–26, 2018, in Paris, France.
Dr Jouvet discloses relationships with the Fonds de Recherche du Québec – Santé, Ministry of Health, Sainte-Justine Hospital, Sage Therapeutic, Medunik, and Covidien. Dr Emeriaud discloses relationships with the Fonds de Recherche du Québec – Santé, and Maquet Critical Care. Dr Fauroux discloses relationships with Association Française contre les Myopathies, Assistance Publique-Hôpitaux de Paris, Inserm, Université Paris Descartes, ADEP Assistance, ASV Santé, S2A Santé, and IP Santé Domicile. The remaining authors have disclosed no conflicts of interest.
Supplementary material related to this paper is available at http://www.rcjournal.com.
See the Related Editorial on Page 614
- Copyright © 2019 by Daedalus Enterprises