Abstract
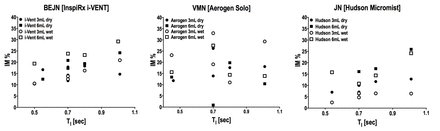
Background: Aerosol technology during modern mechanical ventilation is constantly evolving. This in vitro study compared 3 nebulizer designs: a new breath-enhanced jet nebulizer (BEJN), i-VENT (InspiRx, Somerset NJ); vibrating mesh (VMN), Solo (Aerogen, Galway Ireland); and jet nebulizer (JN), Hudson MicroMist (Teleflex Medical, Morrisville NC) during mechanical ventilation. Aerosol delivery (Inhaled Mass, IM) was measured using multiple ventilator settings (TI = 0.45 to 1.01). Device reproducibility, humidification effects and fill volume were assessed. Measurements included mass balance across the ventilator circuit and particle size distribution. Methods: Using radiolabeled saline and gamma scintigraphy, bench studies were performed using 2 ventilators, 4 breathing patterns. Four examples of each nebulizer type were randomly rotated during testing to assess reproducibility. 3- and 6-mL fill volumes were tested wet and dry [with and without heated wire humidification (Hudson Neptune Heated Humidifier, Teleflex Medical)]. Measurements of IM, nebulizer residual and exhaled mass, plus circuit losses were made to account for the total mass balance. Aerosol particle size distribution (APSD) at the distal ETT tip was made with a Marple cascade impactor. Statistical analysis was performed using Mann-Whitney. Results: Mean ± SD IM (% of nebulizer charge) averaged 17.5 ± 4.99%, 17.2 ± 7.81%, and 6.43 ± 6.43%, for BEJN, VMN and JN respectively (Figure). IM for VMN varied widely due to random failures to complete nebulization. For all ventilator settings, IM for JN was significantly affected by combined effects of fill volume and humidification compared to the BEJN (P = .002) and VMN (P = .02). BEJN and VMN were not different (P = .74). Mass balance indicated that large particles were lost in ventilator tubing for all devices and, for VMN, also in the humidifier (17%), resulting in similar APSD measurements (MMAD = 1.05, 1.05, 1.2µm for BEJN, VMN, and JN). Humidity effects were significant only for JN using 3 mL volume fill (P = .002). Conclusions: Aerosol delivery during BEJN nebulization was less sensitive to fill volume, humidification and ventilator settings than VMN or JN. VMN delivery was not predictable over the range of individual devices and settings. All devices delivered similar particle size distributions to the distal ETT. Breath-enhanced nebulizer technology can ensure better control of drug delivery during mechanical ventilation.
Footnotes
Commercial Relationships: Dr. Ashraf no disclosures. Ms. Cuccia serves as a consultant to InspiRx Inc. Mr. McPeck no disclosures. Dr. Smaldone serves as a consultant and member of the advisory board of InspiRx Inc. Stony Brook University holds patents licensed to InspiRx.
Support: The study was sponsored in part by InspiRx Inc. The State University of New York holds patents on this device that are licensed to InspiRx Inc.
- Copyright © 2019 by Daedalus Enterprises