Abstract
BACKGROUND: ARDS is an overwhelming systemic inflammatory process associated with significant morbidity and mortality. Several trials have evaluated the effects of pharmaconutrients, given as part of a feeding formula or as a nutritional supplement, on clinical outcomes in critical illness and ARDS. The aim of this review is to assess the effects of immunonutrition on mechanically ventilated adults with ARDS compared to the standard feeding formula.
METHODS: We searched MEDLINE, EMBASE, CENTRAL, conference proceedings, and trial registries for appropriate studies up to April 2018. We performed statistical analysis according to Cochrane methodological standards. We used the GRADE approach to assess the quality of evidence for each outcome.
RESULTS: We identified 10 randomized controlled trials with 1,015 participants. All of the studies compared an enteral formula or additional supplemental omega-3 fatty acids (eg, eicosapentaenoic acid, docosahexaenoic acid), γ-linolenic acid, and antioxidants. All of the studies reported mortality. For the primary outcome, there was no difference in all-cause mortality (for the longest period reported) with the use of an immunonutrition enteral formula or additional supplements of omega-3 fatty acids, γ-linolenic acid, and antioxidants (risk ratio = 0.79, 95% CI 0.59–1.07; low-quality evidence). For the secondary outcomes, we are uncertain whether immunonutrition with omega-3 fatty acids and antioxidants improves ICU length of stay, ventilator days, and oxygenation or increases harm.
CONCLUSIONS: This Cochrane meta-analysis of 10 studies of varying quality examined the effects of omega-3 fatty acids and antioxidants in adults with ARDS. This intervention may produce little or no difference in all-cause mortality between groups. We are uncertain whether immunonutrition with omega-3 fatty acids and antioxidants improves ventilator days, ICU length of stay, or oxygenation due to the very low quality of evidence.
Introduction
ARDS is characterized by refractory hypoxic respiratory failure with significant global inflammatory processes and multi-organ dysfunction. In the lung, diffuse epithelial and endothelial injury leads to increased alveolar-capillary permeability and florid pulmonary edema. Clinically, patients present with acute refractory hypoxemia and poor lung compliance, often necessitating invasive mechanical ventilation.1 Hospital mortality associated with ARDS varies between 27% and 45%, depending on the severity of the disease.2,3 Survivors of ARDS have significant long-term physical, cognitive, and psychological sequelae.4,5
Immunonutrition refers to modulation of the immune system provided by specific interventions that modify dietary nutrients.6 It has long been recognized that supplementary immunonutrients may alter the course of critical illness following sepsis, trauma, and surgery.7 Several specialized enteral and parenteral formulas with immunonutrients are currently available on the market. These primarily consist of a combination of antioxidant vitamins (eg, vitamin C, vitamin E, β-carotene), trace elements (eg, selenium, zinc), essential amino acids (eg, glutamine, arginine) or essential fatty acids, such as omega-3 fatty acids (eg, eicosapentaenoic acid, docosahexaenoic acid), and γ-linolenic acid.8 ARDS is characterized by overt recruitment of neutrophils, significant release of pro-inflammatory cytokines and chemokines, and activation of procoagulant cascades and prostaglandin pathways with increased oxidative stress, causing damage to both lipids and proteins.9
In patients with ARDS, a significant imbalance in the antioxidant system with a relative increase in oxidative stress leads to increased alveolar injury.10–12 Among critically ill patients in general, supplementation of antioxidants is associated with a favorable outcome.13 Macronutrients such as glutamine and arginine also have immunomodulatory properties and have been used in several clinical trials of critically ill and surgical patients.14–17 Glutamine improves gut barrier function and can be an energy source for lymphocytes, neutrophils, and macrophages,18,19 whereas arginine deficiency, which is commonly encountered following critical illness, may impair T cell function.20 Omega-3 fatty acids are essential lipids, enriched in fish oil and consisting of polyunsaturated fatty acids such as eicosapentaenoic acid, α-linolenic acid, and docosahexaenoic acid. Therapeutic supplementation of these nutrients, which have immunomodulatory properties, has been shown to moderate the inflammatory response through suppression of pro-inflammatory eicosanoid biosynthesis,21 attenuations of pulmonary neutrophil accumulation,22 reductions in lung permeability,23 and attenuation of cardiopulmonary dysfunction in animal models of lung injury.24 Furthermore, in endotoxemic rat models, eicosapentaenoic acid has been shown to reduce pulmonary edema.25
Various types of immunonutrition have the potential to influence clinical outcomes in critically ill patients.8 Several RCTs have investigated enteral supplementation of omega-3 fatty acids and antioxidants, which, when combined in a meta-analysis, showed a significant reduction in mortality with improvement in oxygenation for patients with acute lung injury and ARDS.26 However, recent randomized controlled trials (RCTs) and subsequent further meta-analyses have presented conflicting results, suggesting a lack of benefit and possibly even harm caused by this intervention.27–31 Among critically ill subjects, enteral supplementation of glutamine conferred no clinical benefit,32 and a large RCT showed a trend toward increased mortality associated with glutamine therapy.16 This lack of demonstrable clinical benefit in recent studies conflicts with the established literature and may be due to the heterogeneity of diseases within the population of critical care patients, or variations in the type, route, and dose of immunonutrients administered. Uncertainty arising from these conflicting results remains. This review aims to provide a comprehensive evaluation of the effects of immunonutrients for patients with ARDS, and to systematically review and critically appraise available evidence on the effects of immunonutrition compared to standard non-immunonutrition formula feeding on mechanically ventilated adults (age ≥18 y) with ARDS.
Methods
This is an abridged version of a previously published Cochrane systematic review and meta-analysis. Details of the methodology are available in the Cochrane version of this systematic review; Cochrane reviews are regularly updated as new evidence emerges and in response to feedback, and the Cochrane Database of Systematic Reviews should be consulted for the most recent version of the review.33 We included all studies involving mechanically ventilated adult participants (age ≥18 y) with ARDS as defined by the Berlin definition of ARDS2 or, for older studies, as defined by American–European Consensus Criteria for both ARDS and acute lung injury.34 Eligible trials included intervention groups consisting of participants given enteral or parenteral immunonutrients, additionally supplemented with or as part of a nutritional formula. In comparison, control groups included participants who received placebo or standard nutrition with a non-immunonutrient formula feed.
Outcome Measures
The primary outcome was all-cause mortality (for the longest period reported). Secondary outcomes were 28-d mortality, ICU length of stay (LOS) and ICU-free days at day 28, ventilator days and ventilator-free days at day 28, hospital LOS, indices of oxygenation (measured as PaO2/FIO2 at day 4 and day 7), other organ failure (ie, a change in organ failure scores: Sequential Organ Failure Assessment [SOFA] score, Multiple Organ Dysfunction Score [MODS]; recorded as the number of subjects with new organ failure developed during the study period), nosocomial infection (ie, additional infection developed during the hospital stay and reported anytime during the study period), and adverse events (eg, author-defined cardiac events, gastrointestinal events, and total adverse events reported anytime during the study period).
Searches, Data Extraction, and Management
We searched on the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 4, April) in the Cochrane Library), MEDLINE (OVID SP; 1966 to April 2018, week 3), and EMBASE (OVID SP; 1988 to April 2018, week 3). Two review authors (AD, RC) independently screened appropriate studies for study characteristics and outcomes. We resolved disagreements by further discussion and with the involvement of a third review author (MG). When necessary, we contacted trial authors to request additional information.
Assessment of Risk of Bias in Included Studies
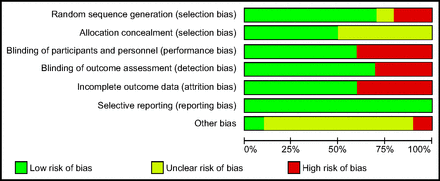
We assessed the risk of bias of included studies according to criteria presented in the Cochrane Handbook for Systematic Reviews of Interventions.35 We assigned the included studies to low risk of bias when all domains were satisfied, high risk of bias if one or more domains were inadequate, or unclear risk of bias, according to the criteria provided in the Cochrane risk of bias tool. We used the following 6 domains to assess the risk of bias in the included studies: selection bias, reporting bias, performance bias, detection bias, attrition bias, and any other bias.
Measures of Treatment Effect
We based the outcome analysis on intention-to-treat. We expressed dichotomous outcomes as risk ratios with 95% CIs, and continuous variables as mean differences with standard deviations. We assessed the clinical heterogeneity of studies in relation to the study population, interventions, and outcome measures. We also assessed inconsistencies and variability in outcomes among studies using the I2 statistic. We assumed substantial statistical heterogeneity when the I2 statistic exceeded 40%.35 We used graphical evidence of reporting biases via contour-enhanced funnel plots with a subsequent Harbord or Egger test.36,37
Subgroup and Sensitivity Analyses
We conducted subgroup analyses for the primary outcome according to type of intervention, route of intervention (parenteral/enteral), mode of intervention (continuous/bolus), and intervention duration (measured in days). We also performed a sensitivity analysis for the primary outcome while excluding studies with high risk of bias. Continuous data for the secondary outcomes were skewed. We conducted a sensitivity analysis for these secondary outcomes by log transformation. Analyses for 2 of the secondary outcomes (ie, ICU-free days and ventilator-free days at day 28) were sensitive to statistical methods; we also performed sensitivity analyses for these outcomes using both fixed-effect and random-effects models to address these issues.
Quality of Evidence
We used the principles of the GRADE system to assess the quality of the body of evidence associated with specific outcomes.38 Through the GRADE approach, we appraised the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. Assessment of the quality of a body of evidence considers the within-study risk of bias (methodological quality), directness of evidence, heterogeneity of data, the precision of effect estimates, and risk of publication bias.
Results
Characteristics of Included Studies
The combined search yielded 3,367 studies for possible inclusion. We retrieved a total of 13 publications reporting 10 RCTs (Fig. 1).27,28,39–46 Three studies were conducted exclusively on subjects with sepsis-induced ARDS.43,44,46 We did not identify any other intervention apart from omega-3 fatty acids (ie, eicosapentaenoic acid and docosahexaenoic acid) and γ-linolenic acid with or without antioxidant-based enteral formula or supplementation. Several clinical trials investigated the use of glutamine supplementation for critical illness, but none specifically focused on ARDS.32
Flow chart.
Among the 10 included studies, 6 of them used a similar enteral preparation (Oxepa; Abbott Nutrition/Abbott Laboratories, Columbus, Ohio) continuously supplemented with eicosapentaenoic acid, docosahexaenoic acid, γ-linolenic acid, and antioxidants (Table 1).36,37,40–43 Four of these 6 studies used an isocaloric high-fat formulation (Pulmocare; Abbott Nutrition/Abbott Laboratories, Columbus, Ohio) for control groups.39,40,43,45 The remaining 2 studies used a carbohydrate-based control formulation (Ensure Plus or Ensure Liquid; Abbott Laboratories, East Windsor, New Jersey).44,46 Three studies used additional enteral supplementation of fish oil,28 omega-3 gels,42 or intravenous formulation with 10% fish oil,41 and control groups received the same enteral feeding formulation as intervention groups. One study gave boluses of a high-fat formulation enriched in eicosapentaenoic acid, docosahexaenoic acid, γ-linolenic acid, and antioxidants and compared this with isocaloric/isovolemic carbohydrate-rich control nutrition.27 Five studies defined a target enteral nutrition delivery rate of 50% to 75% of basal energy expenditure/resting energy expenditure (BEE/REE) for the first 24 h with variable increments to achieve 70% to 100% of BEE/REE.39,40,43–45 The duration of intervention was ≤ 7 d,40,41 14 d,28,41,42,44,45 21 d,27 or 28 d.43,46
Characteristics of Included Studies
Risk of Bias in Included Studies
We identified an increased risk of bias among 6 studies (Fig. 2). Authors of 4 studies reported that they were industry-supported.40,43,45,46 We rated these studies as having an unclear risk of bias.
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
Primary Outcome: All-Cause Mortality (Longest Period Reported)
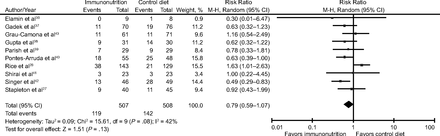
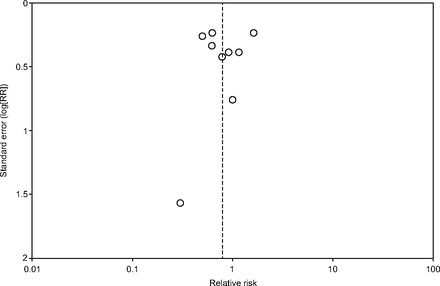
All studies reported mortality. The range of reported mortality varied between studies: six studies reported this outcome at day 28,39,41–43,45,46 three studies at day 60,27,28,44 and one for the study duration40. There was no evidence for the use of omega-3 fatty acids and antioxidants for reducing mortality at the longest period reported (risk ratio = 0.79, 95% CI 0.59–1.07; I2 = 42%; participants = 1,015) (Fig. 3). The pooled control group mortality rate was 28%, and the pooled intervention group mortality rate was 23.5% for the longest period reported. Overall mortality varied between studies and ranged from 6% to 43%. A funnel plot of the primary outcome prepared to test the effect of publication bias showed no evidence of data asymmetry (Egger's regression test, with P = .81) (Fig. 4). We downgraded the quality of evidence by two levels due to inconsistency from clinical and methodological heterogeneity and indirectness for intervention and comparator.
Forest plot for the comparison omega-3 fatty acids and antioxidants versus placebo or standard nutrition. Outcome shown is all-cause mortality (longest period reported).
Funnel plot of comparison: omega-3 fatty acids and antioxidants versus placebo or standard nutrition. Outcome shown is all-cause mortality (longest period reported). No data asymmetry with Egger's regression test (P = .81). RR = relative risk.
Secondary Outcomes
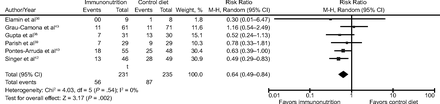
Six studies with 466 participants reported 28-d mortality.39,41–43,45,46 We noted uncertain evidence for use of omega-3 fatty acids and antioxidants in terms of mortality at 28 d (risk ratio = 0.64, 95% CI 0.49–0.84; I2 = 0%) (Fig. 5). This analysis was limited by the small number of participants, which accounted for < 50% of the total participants included.
Forest plot for the comparison omega-3 fatty acids and antioxidants versus placebo or standard nutrition. Outcome shown is 28-d mortality (longest period reported).
There is uncertain evidence for the use of omega-3 fatty acids and antioxidants for the improvements in secondary outcomes of ICU LOS, duration of mechanical ventilation, ICU-free days at day 28 and ventilation-free days at day 28 (Table 2). There was also uncertain evidence for the use of omega-3 fatty acids and antioxidants in terms of improvements in PaO2/FIO2 ratio at day 4 and at day 7, reported new organ failures, nosocomial infections, and adverse events (Table 2). We downgraded the quality of evidence by 3 levels due to increased risk of bias, inconsistency due to clinical and methodological heterogeneity, and indirectness for both intervention and comparator.
Summary Statistical Analysis for Secondary Outcomes
Subgroup Analysis
For the primary outcome, we performed a subgroup analysis for types of control nutrition given, route of administration (parenteral/enteral), mode (continuous/bolus), and the duration of intervention (Table 3). We are uncertain of any mortality benefit when investigators compared an omega-3 fatty acid or antioxidant group with a control group administered with a lipid-rich or carbohydrate-rich formula or as additional supplementation of omega-3 fatty acids. Only one study gave intravenous omega-3 fatty acid supplementation.41 All remaining studies gave enteral supplementation. We are also uncertain of any mortality benefit derived by different routes of administration (enteral or intravenous), either as continuous or bolus supplementation. We are also uncertain of any mortality benefit associated with duration of intervention for < 7 d, 14 d, or for 28 d (Table 3). For all subgroup analyses, we downgraded the quality of evidence by 3 levels due to increased risk of bias, inconsistency due to clinical and methodological heterogeneity, and indirectness for intervention and comparator.
Summary of Subgroup Analysis for Primary Outcomes
Sensitivity Analysis
We conducted a sensitivity analysis for the primary outcome while excluding studies with high risk of bias. We included 4 studies in the analysis.27,28,40,42 We found no evidence for the use of omega-3 fatty acids and antioxidants in reducing mortality at the longest period reported (Table 4). Most data for continuous secondary outcomes, such as ICU LOS, ICU-free days at day 28, ventilator days, ventilator-free days at day 28, and hospital LOS, were skewed. Therefore, we also performed sensitivity analysis for these outcomes from log-transformed data. Despite this transformation, we are uncertain whether this intervention confers any beneficial effect on these secondary outcomes (Table 4). We also performed sensitivity analysis using both fixed-effect and random-effects models for secondary outcomes of ICU-free days and ventilator-free days at day 28 28. Results were sensitive to the type of analytical method used (ie, random-effects model or fixed-effect model) (Table 5).
Sensitivity Analysis for Primary Outcomes*
Sensitivity Analysis for ICU-Free Days and Ventilator-Free Days at Day 28
Discussion
We identified 10 RCTs evaluating effects of omega-3 fatty acids, eicosapentaenoic acid, docosahexaenoic acid, γ-linolenic acid, and antioxidants, given as a supplement or as part of an enteral nutrition formula. We identified no clinical trials with any other specific immunonutrition intervention for this patient population. We found no evidence that this type of nutrition improves the primary outcome of all-cause mortality at the longest period reported. Included studies showed clinical heterogeneity with respect to type, mode, and duration of intervention provided, as well as the type of enteral nutrition formulation received by the control group. We performed subgroup analysis according to different interventions in the control group. Although we noted a statistical reduction in mortality when omega-3 fatty acids and antioxidants were compared with a lipid-rich enteral formula, we are uncertain due to very low-quality evidence whether this intervention improves mortality in the ARDS population. We also found uncertain evidence regarding reductions in duration of mechanical ventilation, ICU LOS, improvement in oxygenation, and increased adverse events with this intervention.
We performed appropriate and thorough searches of electronic databases to identify suitable studies. We applied no restrictions. We obtained additional study details from study authors when possible. Our meta-analysis incorporated 10 clinical trials of 1,015 participants investigating immune-modifying nutrition in subjects with ARDS. All studies used omega-3 fatty acid-based nutritional formula with or without antioxidants for the intervention. However, this approach was subject to significant clinical and statistical heterogeneity. Overall, pooled data did not support giving omega-3 fatty acids in combination with γ-linolenic acid and antioxidants to improve mortality in ARDS. Although results from some subgroup analyses indicate that the mortality risk ratio was reduced when the intervention was given as a continuous enteral infusion against a lipid-rich control formulation, the quality of evidence is very low. Also, the isocaloric high-fat formula used in the control group diet was enriched in omega-6 fatty acids with a high content of linolenic acid, which may have been harmful; in other words, the beneficial effect reported by those studies may have been due to potentially harmful effects of the lipid-rich diet given to the control group.
Given that mechanical ventilator days and ICU LOS may be influenced by the death rate, more recent critical care clinical trials have widely reported ventilator-free and ICU-free days as better outcome measures. In general, ventilator-free days and ICU-free days at day 28 are used as a surrogate for overall ICU outcomes combining mortality with duration of mechanical ventilation and ICU LOS, respectively. Study results show a difference in the pooled statistical analysis between outcomes of ICU LOS and ICU-free days at day 28. This was also true for ventilator days and ventilator-free days at day 28. Reporting of either the number of days with ventilation or in the ICU or numbers days without ventilation or not in the ICU can be subject to bias. For this reason, we meta-analyzed both ways of reporting all available data from published trials (ventilator days/ventilator-free days at day 28 and ICU LOS/ICU-free days at day 28). Alternatively, this disagreement between our secondary composite outcomes (ICU LOS/ventilator days and ICU-free days/ventilator-free days at day 28) and individual end points could be due to inclusion and exclusion of different studies from outcome analyses, based on available outcome measures, or due to lack of standardized reporting. Nevertheless, due to this low-quality evidence, we are uncertain whether the use of an omega-3-based immune-modulating diet in ARDS improves ventilator days or ICU LOS.
Some studies did not provide adequate descriptive evidence for methods of randomization and allocation concealment. One study was unblinded, and 2 studies did not adequately clarify the blinding of outcome assessment. Significant dropouts occurred in several studies for a variety of reasons, including protocol violations and intolerance of the intervention or trial. We graded these studies as having a high risk of bias. Most studies reported anticipated outcomes and all reported mortality, although they were not adequately powered to detect differences between groups. We graded the quality of evidence for the primary outcome as low and for all secondary outcomes as very low because, despite the possibility of increased risk of bias, the primary outcome analysis included > 1,000 subjects with > 250 events and was not influenced by inclusion of studies with high risk of bias, as evidenced by the sensitivity analysis omitting these studies. Analyses of continuous outcome data for most secondary outcomes yielded skewed results. We were not able to obtain the raw transformed data from study authors; therefore, we performed sensitivity analysis using logarithmic transformations for these secondary outcomes. We encountered substantial statistical heterogeneity for several secondary outcomes. Significant clinical and statistical heterogeneity led to a priori defined subgroup analyses of the primary outcomes, according to the type of intervention provided.
Most included studies in this meta-analysis presented indirectness, where there were significant differences between the intervention dietary composition and control formulations. Four RCTs used a comparator enteral formula enriched in omega-6 fatty acids.39,40,43,45 Because omega-6 fatty acids can potentiate inflammation, this may have caused increased harm in the control group. Furthermore, one study gave bolus feeding and reported significant differences between the amount of protein given per day to the control group (20 g) and the intervention group (4 g).27 There were also differences in the patient population between included studies, in that 3 studies exclusively consisted of subjects with sepsis-related ARDS.43,44,46 Etiological heterogeneity of ARDS origin may have different pathophysiological consequence, and modulation of inflammation by immunonutrition in sepsis and septic shock has produced conflicting results.47,48 Consequently, combining these studies of both pulmonary and extrapulmonary origin of subjects with ARDS may have introduced clinical heterogeneity and thus bias. Overall, we rated the quality of evidence as low to very low because of increased risk of bias, skewed data, inconsistency, and indirectness. Further research on this topic is essential both to address the overall objective of this review and to focus on specific questions. Future RCTs should consider standardized reporting of ICU outcomes to facilitate the combination and comparison of data between studies.
To our knowledge, we have identified and included all published studies on this topic. Lack of standardization in reporting outcomes resulted in some studies reporting duration of ICU stay as ICU LOS and others as ICU-free days at day 28. We encountered similar issues when dealing with duration of mechanical ventilation. This resulted in pooling of these studies separately, which was not planned when the protocol was drafted. Lack of standardized statistical data from the included studies led to assumptions of normal distribution and calculations of standard deviation from standard error, interquartile ratio, and P value, which may have introduced additional bias.
Pontes-Arruda et al26 conducted the first meta-analysis on this topic in 2008. This review included 3 earlier studies40,43,35 and reported a survival advantage, improvement in oxygenation (at day 4 and at day 7), and other clinical variables such as ICU-free days and ventilator-free days at day 28 with immune-enhancing diets.26 All studies included in this review gave a high-fat, low-carbohydrate formula enriched with eicosapentaenoic acid, γ-linolenic acid, and antioxidants. However, the beneficial effects seen in these trials may have been confounded by increased mortality in the control groups, possibly caused by the use of enteral formula enriched in omega-6 fatty acids. Another meta-analysis, which only included these 3 earlier studies and focused on mortality and oxygenation, yielded the same conclusions.29
Results from a subsequent meta-analysis of 7 studies contradicted these previous positive findings and revealed that enteral supplementation of omega-3 fatty acids, γ-linolenic acid, and antioxidants provide no benefit in reducing clinical outcomes such as 28-d mortality, ventilator-free days, and ICU-free days.30 However, these results showed an improvement in oxygenation at day 4 and at day 7 with immune-modulating diets. In comparison to the previous meta-analyses, this review included 4 additional studies, and 3 of these were methodologically different from the older studies.27,28,46 The largest of these trials (ie, OMEGA Trial) demonstrated no mortality benefit and was terminated early due to futility after an interim analysis. In contrast to the older studies, the intervention in this trial consisted of twice-daily bolus supplementation of omega-3 fatty acids, γ-linolenic acid, and antioxidants or the control supplement composed of an isocaloric, high-carbohydrate, low-fat feed.27 Another study supplemented enteral fish oil,28 whereas another gave a lipid-based diet with omega-3 fatty acids, γ-linolenic acid, and antioxidants as the intervention and used a high-carbohydrate, lipid-poor control enteral feed.46 A more recent meta-analysis of 6 RCTs31 assessed the effect of enteral omega-3 fatty acids, γ-linolenic acid, and antioxidants. They demonstrated significant heterogeneity across studies and found no difference in clinical outcomes such as all-cause mortality, ventilator-free days, or ICU-free days.31 In this review, the authors also analyzed studies with high overall mortality and demonstrated a positive outcome with the intervention for patient groups with higher mortality, inferring potential benefit for those with severe ARDS.
Our meta-analysis was consistent with the conclusions of recently published reviews in finding no mortality benefit derived from immunomodulatory diets based on the inclusion of omega-3 fatty acids, γ-linolenic acid, and antioxidants. We noted no improvement in ventilator-free days or ICU-free days at day 28, and this was sensitive to analytical methods. These findings were consistent with those reported by a previous meta-analysis.30 Our findings are also consistent with guidelines of the Society of Critical Care Medicine and the American Society for Parenteral and Enteral Nutrition for the provision of nutritional support for adult critically ill patients, which do not recommend the use of omega-3 fatty acids in the ARDS population.49
Our systematic review and meta-analysis showed no mortality benefit associated with use of immunonutrition in ARDS populations. Current data do not justify a large RCT on this topic but would support targeted proof-of-concept studies in groups of subjects to refine the intervention. Consistent reporting of outcome measures by researchers will be important to allow combinations of results in subsequent meta-analyses. Mortality is unlikely to be the best outcome measure for such studies. Cost-effectiveness data are notably absent from current studies and should be collected in the future. The most promising areas for future evaluation include continuous supplementation with a balanced formula for both control and intervention groups with additional supplementation for the intervention group.
Conclusions
This meta-analysis evaluated 10 heterogeneous studies of varying quality and analyzed effects of omega-3 fatty acids and antioxidants in critically ill adults with ARDS. We did not find clinical trials of any other immunonutrition intervention provided to this patient group. Despite the inclusion of all studies in our meta-analysis, we were not able to pool all studies for every anticipated clinical outcome due to the lack of standardized outcome reporting. This may have introduced bias into our analysis. Our results suggest that no mortality benefit is derived from the use of omega-3 fatty acids or antioxidants in ARDS. Uncertain evidence suggests reductions in duration of mechanical ventilation and ICU LOS, along with improved oxygenation. The quality of evidence was very low due to several factors, including poor-quality small trials with high risk of bias, clinical and methodological heterogeneity, and issues due to imprecision and inconsistency between trials, with additional indirectness due to an imbalance in nutrition provided to the comparator groups.
Acknowledgments
We thank Karen Hovhannisyan for her help in designing the searches, and Jane Cracknell, Managing Editor of the Cochrane Anaesthesia Critical and Emergency Care Group, for her assistance. We thank Bronagh Blackwood (content editor), Nathan Pace (statistical editor), Davoud Vahabzadeh and Thomas Bongers (peer reviewers), Patricia Tong (consumer referee), and Harald Herkner (coordinating editor) for their help and advice provided during the preparation of this systematic review. We also thank Rodrigo Cavallazzi (content editor), Jing Xie (statistical editor), and Todd Rice and Davoud Vahabzadeh (peer reviewers) for their help and editorial advice provided during preparation of the protocol for this systematic review.
Footnotes
- Correspondence: Ahilanandan Dushianthan PhD, General Intensive Care Unit, University Hospital Southampton NHS Foundation Trust, Tremona Road, Southampton, UK SO16 6YD. E-mail: adushianthan{at}gmail.com.
Dr Grocott has disclosed relationships with Baxter, BOC Medical (Linde Group), Eli Lilly Critical Care, Fresenius-Kabi, Smith Medical, Deltex Medical, London Clinic, and Rolex. Dr Calder has disclosed relationships with Abbott Nutrition, Baxter Healthcare, Danone/Nutricia, Fresenius-Kabi, Pronova BioPharma/BASF AS, and Smartfish. The other authors have disclosed no conflicts of interest.
This article is based on a Cochrane Review published in the Cochrane Database of Systematic Reviews (CDSR) 2019, Issue 1, DOI: 10.1002/14651858.CD012041 (see www.thecochranelibrary.com for information). Cochrane Reviews are regularly updated as new evidence emerges and in response to feedback, and the CDSR should be consulted for the most recent version of the review.
- Copyright © 2020 by Daedalus Enterprises