Abstract
BACKGROUND: High-flow nasal cannula (HFNC) is commonly used to provide respiratory support to pediatric patients with respiratory failure. Although the use of bronchodilators via HFNC has been described, the feasibility and safety of aerosolized bronchodilator delivery via HFNC are controversial. In this study, we sought to evaluate whether the HFNC system can be used to deliver nebulized bronchodilators at lower gas flow of 2–4 L/min, increase patient comfort, and minimize respiratory therapist (RT) bedside time when compared to traditional interfaces.
METHODS: A retrospective chart review of all pediatric subjects who were admitted to the pediatric ICU in a tertiary care children’s hospital and required nebulized bronchodilators between December 2017 and June 2018.
RESULTS: A total of 205 nebulizations were administered to 28 children; 31% of nebulized bronchodilators were given using a nebulization system integrated into the HFNC. Nebulized treatments resulted in an average increase in heart rate of 9.98 (95% CI 3.72–16.2) beats/min when HFNC was used and 0.64 (95% CI −1.65 to 2.93) beats/min when traditional interfaces were used, a difference of 9.34 (95% CI 2.30–16.4) beats/min (P < .001). RT bedside time was significantly longer for HFNC nebulized treatments (P = .031). Subjective level of comfort was not statically different when nebulized bronchodilators were delivered via HFNC or via traditional interfaces. Length of pediatric ICU stay was not statistically different between subjects who received some aerosol nebulized bronchodilators via HFNC versus those who received all bronchodilators through traditional interfaces (P = .11).
CONCLUSIONS: Aerosol bronchodilator delivery using HFNC is feasible at low gas flow (ie, 2–4 L/min). However, the use of HFNC did not improve subjects’ comfort, and it increased RT bedside time. Further prospective randomized studies are needed to determine the efficacy and efficiency of aerosol therapy delivered through HFNC and potential patient-oriented outcomes.
Introduction
High-flow nasal cannula (HFNC) is commonly used to provide respiratory support to pediatric patients with respiratory failure.1-4 Traditionally, gas flow ≥2–4 L/min was considered high flow, but flows up to 2–3 L/kg body weight/min are being used in children to deliver higher concentrations of oxygen and provide increased respiratory support via continuous distending pressure or dead space washout.5-8
Aerosolized bronchodilators are often used effectively to manage critically ill children supported with HFNC.9,10 Options to deliver aerosolized bronchodilators to these patients are limited to the use of a mask and either removing the HFNC or delivering nebulized bronchodilators with a mask while keeping the HFNC in place. Both of these options, however, are potentially unsafe; the former requires interruption of HFNC support, and the latter may prevent the delivery of the bronchodilator. Both methods may also lead to higher patient anxiety and could be time-consuming. Therefore, the use of HFNC to nebulize bronchodilators may be a convenient and attractive option.11 Although the use of bronchodilators via HFNC has been described, the efficiency of the therapy and aerosolized bronchodilators delivery are controversial because it is unclear whether clinically relevant doses of the aerosol make it to the targeted receptors in the airways. In a recently published review, we found that the amount of aerosol delivery is low at high gas flow, but it may increase when using flows of 2–4 L/min.4,9,12-14 Therefore, we recently standardized selection of aerosol delivery interfaces for patients requiring HFNC in our institution. In this study, we retrospectively investigated whether the HFNC can be used to deliver nebulized bronchodilators at low gas flow (ie, 2–4 L/min), improve subject comfort, and minimize respiratory therapist (RT) bedside time compared to traditionally used delivery methods.
QUICK LOOK
Current knowledge
High-flow nasal cannula (HFNC) is commonly used to provide respiratory support to critically ill children with respiratory failure. At times, aerosolized bronchodilators might be needed to manage pediatric patients supported with HFNC.
What this paper contributes to our knowledge
Aerosolized bronchodilator delivery using HFNC was feasible at low gas flow (ie, 2–4 L/min), but the use of HFNC to deliver bronchodilators might increase respiratory therapists’ bedside time without improving patient comfort or satisfaction.
Methods
The Institutional Review Board at the University of Wisconsin-Madison approved the study protocol with a waiver of informed consent (Protocol ID: 2018-1393). The charts of pediatric subjects who were admitted to the pediatric ICU and required nebulized bronchodilators at American Family Children’s Hospital between December 2017 and June 2018 and were part of a quality-improvement initiative to standardize aerosol delivery were included in the study. Patients who were intubated upon pediatric ICU admission were excluded. The admission diagnosis as well as the reason for prescribing bronchodilators were obtained from medical charts. During the study period, our unit used the Optiflow Junior system (Fisher & Paykel, Auckland, New Zealand) with a humidifier (MR850, Fisher & Paykel) on the dry end of the circuit (Fig. 1). We also used Aerogen Solo (Aerogen, Galway, Ireland) nebulizer with palladium vibrating mesh technology. We used the KidsMED face mask (Vyaire/CareFusion, California), and we used the Trilogy 202 or V60 (Philips Respironics, Best, Netherlands) for bi-level positive airway pressure ventilation with the Aerogen Solo palladium vibrating mesh nebulizer. All nebulizers were placed on the dry end of the circuit because recent literature suggest that nebulizer placement on the dry end of the circuit increases deposition of aerosol nebulized bronchodilators.15 The interface selection for delivery of aerosolized bronchodilators was based on our newly developed guidelines and were age-appropriate based on the manufacturers recommendations (Fig. 2). All non-HFNC nebulized treatments were broadly labeled as traditional interfaces. Traditional interfaces included face mask, pacifier nebulizer, continuous noninvasive positive airway pressure or bi-level positive airway pressure circuits. For subjects on HFNC, RTs weaned flows down to 2–4 L/min prior to nebulizing bronchodilators. Subjects who didn’t tolerate weaning flows and required > 4 L/min were switched to traditional interfaces. The following were recorded by the RT before and after each nebulized treatment: heart rate (beats/min), breathing frequency (breaths/min), pulse oximetry (%), 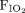 , RT time at the bedside (min), life-threatening serious adverse events, and comfort assessments. Subjects were considered comfortable if they were calm, not crying, and had no facial tensions or aggressive physical movements. Heart rate response was used as an indirect assessment of bronchodilator delivery.
, RT time at the bedside (min), life-threatening serious adverse events, and comfort assessments. Subjects were considered comfortable if they were calm, not crying, and had no facial tensions or aggressive physical movements. Heart rate response was used as an indirect assessment of bronchodilator delivery.
High-flow nasal cannula apparatus used during the study period. The mesh nebulizer was located on the dry side of the humidifier. (1) Mesh nebulizer control; (2) air-oxygen blender and flow meter; (3) pressure pop off valve; (4) Mesh nebulizer; (5) water chamber; (6) heated inspiratory circuit; (7) active humidifier; (8) nasal cannula.
Pediatric ICU bronchodilator interface selection guidelines. BPAP = bi-level positive airway pressure; HFNC = high-flow nasal cannula; MDI = metered-dose inhaler; SVN = small-volume nebulizer; BAN = breath-actuated nebulizer.
Statistical Analysis
The entire cohort of children was summarized with frequencies and percentages for categorical variables and with medians and interquartile range (IQR) for numerical characteristics. Comparisons of these characteristics by HFNC use (ever vs never) were made using chi-square (categorial variables) or rank-sum tests (numerical variables). A negative binomial distribution (using length of pediatric ICU stay as an offset) was used to model the mean number of nebulized treatments per child per 24 h spent in the pediatric ICU. To assess whether either the number of nebulized treatments or the length of pediatric ICU stay was associated with a patient having ever been put on HFNC, both models were extended to include HFNC use (ever vs never) as a covariate. Heart rate, breathing frequency,  , and flows were measured before and after each nebulized treatment, and analyses were conducted to determine whether a change in any of these responses was associated with having been on HFNC at that point in time. Due to repeat nebulization measurements within each child, generalized estimating equations were used to make comparisons involving the degree of change using HFNC or standard delivery methods (ie, traditional interfaces).16,17 Generalized estimating equations models were also used to explore dichotomized changes in comfort, defined as subjects who were calm before treatment and remained calm or subjects who were agitated before the treatment and then became calm, before and after each nebulized treatment to test for differences in HFNC versus traditional interfaces with respect to RT time and to understand whether these changes in heart rate, breathing frequency, and
, and flows were measured before and after each nebulized treatment, and analyses were conducted to determine whether a change in any of these responses was associated with having been on HFNC at that point in time. Due to repeat nebulization measurements within each child, generalized estimating equations were used to make comparisons involving the degree of change using HFNC or standard delivery methods (ie, traditional interfaces).16,17 Generalized estimating equations models were also used to explore dichotomized changes in comfort, defined as subjects who were calm before treatment and remained calm or subjects who were agitated before the treatment and then became calm, before and after each nebulized treatment to test for differences in HFNC versus traditional interfaces with respect to RT time and to understand whether these changes in heart rate, breathing frequency, and  were associated with changes in comfort or wakefulness. All analyses were performed using R 3.5.1 (R Foundation, Vienna, Austria) and the geepack package.18,19
were associated with changes in comfort or wakefulness. All analyses were performed using R 3.5.1 (R Foundation, Vienna, Austria) and the geepack package.18,19
Results
A total of 28 children received nebulized therapy between December 2017 and June 2018: 60.7% were male, median age was 32.4 months (IQR 15.9–53.2), and the median weight was 13.5 kg (IQR 10.4–18.1). Asthma was the most prevalent admitting diagnosis, followed by bronchiolitis (Table 1). Median time spent in the pediatric ICU was 31.8 h (IQR 19.6–68.8), and children received on average 4.2 (95% CI 3.4–5.3) nebulizations per 24 h; 57% of children never received bronchodilators through HFNC during their stay in the pediatric ICU, and the remainder received at least one aerosol therapy using HFNC. Two subjects (7.1%) didn’t tolerate weaning flows to 2–4 L/min and were switched to traditional interfaces. Table 1 shows that length of pediatric ICU stay was not statistically different (P = .11) between subjects who received some aerosol therapy via HFNC (22.7 h [IQR 18.0–50.6]) or traditional interfaces (46.7 h [IQR 25.4–77.2]).
Demographics and Variables for the Study Cohort
A total of 205 nebulized treatments were administered to the 28 pediatric subjects; 31.2% were given using a nebulization system integrated into the HFNC, and 68.8% were given using traditional interfaces. The median flow immediately prior to the nebulized treatment was 7 L/min (IQR 4–10). Nebulized treatments resulted in an increase in heart rate by an average of 9.98 (95% CI 3.72–16.2) beats/min when HFNC was used and 0.64 (95% CI −1.65 to 2.93) beats/min when traditional interfaces were used. The difference in heart rate increase between the groups was statistically significant (P < .001), with the increase being 9.34 (95% CI 2.30–16.4) beats/min greater for HFNC than traditional interface. When on HFNC, breathing frequency after nebulization increase by 1.49 (95% CI −0.05 to 3.04) breaths/min after nebulized treatment. However, there was no statistically significant increase in breathing frequency after nebulization when traditional interfaces were used (ie, 0.02 [95% CI −1.64 to 1.68], P = .26). Similar conclusions were reached concerning changes in 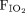 levels (Table 2).
levels (Table 2).
Changes in Subjects’ Vital Signs and Comfort
Improvement in the level of comfort after nebulized treatments occurred in 87.9% of HFNC treatments and in 82.9% of traditional interfaces (P = .56). RT time at bedside was significantly longer for HFNC treatments (P = .031), with the average number of minutes for HFNC treatments estimated to be 26% (95% CI 2–56%) greater than the mean RT time for other treatments: 14.6 min vs 11.6 min; estimated absolute increase of 3 (95% CI 0.11–5.9) min (Table 2). Secondary analysis showed that heart rate, breathing frequency, and 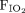 changes when treatments were delivered using HFNC versus traditional interfaces were not influenced by the level of comfort and wakefulness (Table 3). Finally, we didn’t observe any life-threatening serious adverse events such as emergency intubation or cardiac arrest.
changes when treatments were delivered using HFNC versus traditional interfaces were not influenced by the level of comfort and wakefulness (Table 3). Finally, we didn’t observe any life-threatening serious adverse events such as emergency intubation or cardiac arrest.
Changes in Subjects’ Vital Signs, Comfort, and Wakefulness*
Discussion
In this study, we report that using HFNC to deliver nebulized bronchodilators is feasible at flows of 2–4 L/min when compared to traditional interfaces. This was supported by our observation that heart rate increased significantly after each nebulized treatment delivered by HFNC and that the mean length of pediatric ICU stay was not statistically different between subjects who received aerosol therapy via HFNC or traditional interfaces. However, the use of HFNC to deliver nebulized bronchodilators did not improve subjects’ comfort, nor did it minimize respiratory therapists’ bedside time. To our knowledge, this study is the largest clinical report of aerosolized bronchodilator delivery via HFNC using low flows to ensure delivery of nebulized bronchodilators.
Aerosolized bronchodilator delivery using HFNC remains controversial. In vitro evidence suggests that aerosol particle delivery is only feasible at gas flows < 4 L/min, and there is little in vivo evidence of its effectiveness at any flow. To our knowledge, there is only one pediatric case series and one randomized crossover study that investigated the use of HFNC to deliver bronchodilators in critically ill pediatric patients.20,21 Similar to our study, those two studies focused on feasibility and comfort. However, both studies delivered bronchodilator without adjusting gas flow to optimize aerosol particles delivery. Our results are consistent with those reported by Morgan et al,20 who described the use of HFNC to deliver aerosolized bronchodilators in 5 infants with acute bronchiolitis and respiratory distress. They reported that bronchodilators delivered via HFNC resulted in greater heart rate increase, which is an indirect assessment of bronchodilator clinical effect. However, Morgan et al20 used gas flows of 5–8 L/min and did not adjust gas flow during treatments. Moreover, they did not assess length of pediatric ICU stay or any other patient-oriented outcomes. In contrast, our results indicate that the use of HFNC to deliver bronchodilators did not result in longer pediatric ICU length of stay or cause any life-threatening serious adverse events.
Patient comfort is considered an important indicator of quality of care and might affect outcomes.21 Therefore, the use of HFNC to nebulize bronchodilators becomes an attractive option because it offers a convenient delivery method with minimal patient manipulation. Using the COMFORT-Behaviour scale, Valencia-Ramos et al21 evaluated the comfort and satisfaction of bronchodilator aerosol delivery using a HFNC in 6 infants with bronchiolitis. They reported that bronchodilators delivered using HFNC resulted in an increased level of comfort and satisfaction compared to conventional jet nebulizers. In contrast to their findings, however, our results did not suggest that subjects’ comfort improved when using HFNC compared to traditional interfaces. Our results might be explained by the fact that RTs caring for our subjects did not use an objective comfort scale to assess subjects before, during, and after treatments.
In a recent survey, Miller et al10 found that 75% of surveyed RTs used HFNC to deliver aerosol therapy. Although the authors didn’t comment on the reasons why a high percentage of responders used HFNC despite a lack of clinical evidence, possible explanations include potential improvement in subject comfort and family satisfaction, or the convenience of HFNC to reduce RTs’ bedside time and effort in delivering the nebulized therapies. However, our results indicate that the use of HFNC significantly increased RTs bedside time. This could be explained by our RT practice of observing patient tolerance to the weaning of gas flow for 2–3 min prior to the initiation of each nebulized treatment. In addition, the RT must remove the vibrating mesh nebulizer from the HFNC circuit, return flow to baseline, validate that the HFNC system is functioning properly, and secure the cannula upon completion of the aerosol treatment. It is also our practice to have continuous assessment of a patient’s response to nebulized bronchodilators and the documentation required for each treatment.
Finally, the delivery of aerosol particles is a very complicated process and could be affected by many factors. Some of those factors are patient-specific, such as age, airway anatomy, breathing effort, and pattern, and others depend on the nebulizer and the interface.4,9,22 In our study, subjects who received bronchodilators through HFNC were younger than subjects who received bronchodilators using traditional interfaces, which might have affected aerosol delivery given that flow, resistance, and turbulence decrease with younger age.23 Amirav et al24 assessed aerosol delivery using pediatric airway models of infants and toddlers and reported that nasal aerosol delivery to the lower respiratory tract was higher than oral delivery in models for infants and young toddlers. However, that difference diminished with age and became negligible when a 20-month-old toddler model was used, which is younger than the median age our HFNC subjects (Table 1). In addition, we used the Optiflow Junior system (Fisher & Paykel) with a humidifier and vibrating mesh nebulizer placed on the dry end of the circuit. The choice of this setup was based on extensive review of the available in vitro and in vivo literature. Therefore, our results might not be applicable when other HFNC systems are used or if mesh nebulizers are placed on the wet end of the circuit or closer to patient’s face. Future clinical studies should focus on the safety and feasibility of using other HFNC systems and the optimum location of the nebulizer in the HFNC circuit.
Our study has several limitations. First, it is a retrospective study with a relatively small sample size. Second, comfort levels were not assessed using an objective comfort scale. Third, although our unit’s guidelines for aerosol delivery were based on current literature, this method has not been validated. Finally, our study had a heterogeneous patient population with different pathophysiology that might affect the response to bronchodilator therapies.
Conclusions
This retrospective study indicates that aerosolized bronchodilator delivery using HFNC was feasible when using low gas flows. However, the use of HFNC did not improve stay, subjects’ comfort, or minimize RTs’ bedside time. Further prospective randomized studies are needed, not only to determine aerosol deposition and effectiveness, but also to ascertain subject safety and outcomes when aerosol therapy is delivered through HFNC at different gas flows.
Footnotes
- Correspondence: Awni M Al-Subu MD, Division of Pediatric Critical Care, Department of Pediatrics, 600 Highland Ave, Room H6/535 CSC, Madison, WI 53792. E-mail: al-subu{at}pediatrics.wisc.edu
The authors have disclosed no conflicts of interest.
- Copyright © 2020 by Daedalus Enterprises









