Abstract
BACKGROUND: Pediatric ARDS is a heterogeneous disease entity with high morbidity and mortality. In this study, we categorized pediatric ARDS by direct and indirect initial triggering events and identified characteristics of survivors and nonsurvivors in these 2 subtypes.
METHODS: This was a single-center, retrospective, observational study that included critically ill subjects with pediatric ARDS (age 1 month to 18 y) who had undergone mechanical ventilation support and had been admitted to our 14-bed, multidisciplinary, tertiary pediatric medical ICU between January 2010 and March 2019.
RESULTS: A total of 162 subjects with pediatric ARDS were included. The direct ARDS subtype accounted for 128 cases, and 34 cases were classified as indirect ARDS. The most common initiating events were pneumonia and sepsis for direct and indirect ARDS, respectively. Subjects with indirect ARDS had higher serum lactate levels, greater Pediatric Risk of Mortality III (PRISM III) and Pediatric Sequential Organ Failure Assessment (pSOFA) scores than those with direct ARDS (P < .05). Nonsurvivors with the direct subtype had worse mechanical ventilation-related parameters, including 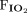 , PEEP,
, PEEP, 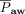 , peak inspiratory pressure, oxygenation index, and
, peak inspiratory pressure, oxygenation index, and 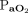 /
/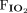 ratio than survivors with the direct subtype. The likelihood of mortality rose with the severity of ARDS in association with the direct subtype but not with the indirect subtypes. Among children with indirect ARDS, lactate levels and pSOFA scores were significantly higher among nonsurvivors than survivors.
ratio than survivors with the direct subtype. The likelihood of mortality rose with the severity of ARDS in association with the direct subtype but not with the indirect subtypes. Among children with indirect ARDS, lactate levels and pSOFA scores were significantly higher among nonsurvivors than survivors.
CONCLUSIONS: Direct and indirect pediatric ARDS had distinct clinical characteristics, especially in terms of prognostic factors. Variables related to mechanical ventilation were significantly associated with mortality among subjects with direct pediatric ARDS, but not among subjects with indirect pediatric ARDS. Thus, this study provides evidence of the potential benefit of categorizing patients with pediatric ARDS by subtype for evaluating prognostic factors and developing adjusted management strategies to improve clinical outcomes.
Introduction
Pediatric patients with ARDS differ from adult patients with ARDS in terms of characteristics such as etiologic factors, pathophysiology, clinical course, and outcomes.1-7
Since ARDS was first described in 1967,8 numerous studies have been performed addressing various clinical issues including definitions, diagnostic criteria, classification, risk factors for mortality, and therapeutic strategies to improve clinical outcomes.9-16 However, such investigations have mainly addressed adult ARDS. In 2015, the Pediatric Acute Lung Injury Consensus Conference (PALICC) established a definition for pediatric ARDS, which took into consideration the differences between adult and pediatric ARDS.3 However, due to the relative rarity of pediatric ARDS, the diversity between study populations, and the associated low mortality, it has been challenging to conduct well-designed, large-scale, clinical trials.17-20 This has hindered the elucidation of characteristics and the development of evidence-based management guidelines for pediatric ARDS. As a result, most clinical therapeutic interventions have been derived from studies of adult ARDS.
Despite many efforts to offer proper interventions, pediatric ARDS remains a challenging disease with high morbidity and mortality. Researchers have become increasingly interested in attributing the disease’s intractability to the heterogeneity of ARDS. Consequently, there have been growing efforts to categorize ARDS into subtypes according to various characteristics, including classifying ARDS as being caused either by a direct lung injury or by an indirect lung injury.21-28 This approach could facilitate the identification of distinct features, according to subtype, in terms of pathophysiology, radiologic findings, respiratory mechanics, responses to management, and clinical outcomes among patients with ARDS.29-35
In this study, we aimed to judge the efficacy of categorizing pediatric ARDS into direct and indirect subtypes in improving homogeneity among study populations and characterization of pediatric ARDS. To this end, subjects in this study were classified as having either direct or indirect pediatric ARDS and were compared in terms of clinical features, with emphasis placed on investigating the differences in clinical features between survivors and nonsurvivors.
QUICK LOOK
Current knowledge
ARDS is a complex syndrome with multiple risk factors and heterogeneous clinical phenotypes. Recently, there have been efforts to categorize ARDS into direct and indirect ARDS, which have distinct features. However, such investigations have mainly addressed adult ARDS.
What this paper contributes to our knowledge
We compared direct and indirect subtypes of pediatric ARDS, which showed different characteristics particularly in terms of prognostic factors. With direct pediatric ARDS, respiratory variables were significantly associated with mortality, but this was not the case with indirect pediatric ARDS.
Methods
Subjects
This study was approved by the institutional review board of Asan Medical Center. The informed-consent requirement was waived due to the retrospective nature of the study. All critically ill children consecutively admitted to the 14-bed multidisciplinary pediatric medical ICU of our hospital between October 2010 and March 2019 were eligible for enrollment. Among these patients, we included subjects age 1 month to 18 y who received endotracheal intubation and mechanical ventilation support for respiratory failure for ≥ 48 h in the pediatric ICU, and who met the Pediatric Acute Lung Injury Consensus Conference (PALICC) definition for pediatric ARDS.3 Subjects were excluded from the study if they received noninvasive ventilatory support, were < 1 month old or > 18 y old, had mechanical ventilation support postoperatively or due to heart failure, had congenital heart diseases with right to left shunting, or had do-not-resuscitate orders.
We then classified all subjects as having either direct or indirect ARDS based on their concurrent medical records. The direct ARDS group included subjects who had experienced a primary insult to the lung, such as pneumonia or aspiration. The indirect pediatric ARDS group included subjects with causes such as sepsis, pancreatitis, or multiple transfusions.
Data Collection
We retrospectively reviewed the electronic medical records of all enrolled subjects. We collected information on subjects’ basic demographics, medical comorbidities, pediatric ICU management, and clinical outcomes. We also reviewed mechanical ventilation-related parameters, including  , PEEP,
, PEEP, 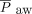 , peak inspiratory pressure, driving pressure (ie, difference between peak inspiratory pressure and PEEP), and tidal volume on day 1 of diagnosis. From the mechanical ventilation-related variables, we calculated
, peak inspiratory pressure, driving pressure (ie, difference between peak inspiratory pressure and PEEP), and tidal volume on day 1 of diagnosis. From the mechanical ventilation-related variables, we calculated 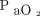 /
/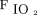 and the oxygenation index [(
and the oxygenation index [(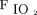 ×
×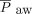 × 100)/
× 100)/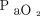 ]. Subjects were classified into mild, moderate, and severe pediatric ARDS groups according to the PALICC oxygenation index criteria at the time of pediatric ARDS diagnosis. We also retrieved arterial blood gas analysis findings (ie, white blood cell, neutrophil, and platelet counts), as well as albumin, creatinine, and C-reactive protein levels from data collected at the time of ARDS diagnosis. We calculated disease severity using the Pediatric Risk of Mortality III (PRISM III)36 and the Pediatric Sequential Organ Failure Assessment (pSOFA) scores37 collected at the time of diagnosis.
]. Subjects were classified into mild, moderate, and severe pediatric ARDS groups according to the PALICC oxygenation index criteria at the time of pediatric ARDS diagnosis. We also retrieved arterial blood gas analysis findings (ie, white blood cell, neutrophil, and platelet counts), as well as albumin, creatinine, and C-reactive protein levels from data collected at the time of ARDS diagnosis. We calculated disease severity using the Pediatric Risk of Mortality III (PRISM III)36 and the Pediatric Sequential Organ Failure Assessment (pSOFA) scores37 collected at the time of diagnosis.
Outcomes
The primary outcome was 28-d mortality, which was defined as death within 28 d of diagnosis of pediatric ARDS. Secondary outcomes included duration of mechanical ventilation support, ventilator-free days, length of stay in the pediatric ICU, and length of hospital stay.
Statistical Analysis
Data were analyzed with SPSS Statistics for Windows 21.0 (IBM, Armonk, New York). Continuous and categorical variables were summarized as medians with interquartile ranges or frequencies with corresponding percentages, respectively. We used the chi-square or the 2-tailed Fisher exact tests to compare qualitative categorical variables between groups. For comparing continuous quantitative variables, the unpaired Student t test was used if the data were normally distributed, and the nonparametric Mann-Whitney U test was used if the variables demonstrated a non-normal distribution. For all analyses, a 2-tailed P < .05 was considered statistically significant.
Results
Subject Characteristics
A total of 162 subjects were included. The clinical characteristics of the subjects are presented in Table 1. There were 87 boys and 75 girls, and the median age and weight at the time of ARDS diagnosis were 2.3 (0.7–7.1) y and 10.0 (5.7–18.3) kg, respectively. Sixty-seven subjects were diagnosed with mild pediatric ARDS, 56 with moderate pediatric ARDS, and 39 with severe pediatric ARDS. A total of 128 subjects were classified as having direct pediatric ARDS, and 34 subjects were classified as having indirect pediatric ARDS. The most frequently detected underlying diseases were neurologic disorders in 40 (24.7%) subjects, followed by oncologic disorders in 34 (21.0%) subjects. The overall mortality rate was 16.0%.
Baseline Clinical Characteristics of Subjects
Comparison Between Direct and Indirect Pediatric ARDS
Pneumonia (89.0%) and sepsis (94.1%) were the most common causes of direct and indirect ARDS, respectively. Compared with subjects with direct ARDS, those with indirect ARDS had higher serum lactate and creatinine levels, lower platelet count, lower driving pressure, and higher PRISM III and pSOFA scores than those with direct ARDS (P < .05; Table 2). The mortality rate was higher in association with indirect than with direct ARDS (26.5% vs 13.3%), but this difference was not statistically significant (P = .059).There were no significant differences in the duration of mechanical ventilation support, length of stay in the pediatric ICU, or the length of hospital stay between subjects with indirect and direct ARDS. However, subjects with indirect ARDS had significantly fewer ventilator-free days than those with the direct subtype (P < .001).
Mechanical Ventilation–Related Parameters and Lab Findings of Subjects With Direct and Indirect Pediatric ARDS
Comparison Between Survivors and Nonsurvivors
Among subjects with direct ARDS, mechanical ventilation–related variables (eg, 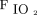 , PEEP, peak inspiratory pressure,
, PEEP, peak inspiratory pressure,  , and oxygenation index) were significantly higher among nonsurvivors than survivors. Among subjects with indirect ARDS, mechanical ventilation-related parameters did not differ significantly between nonsurvivors and survivors, whereas serum lactate levels and pSOFA scores were significantly higher among nonsurvivors compared to survivors (Table 3).
, and oxygenation index) were significantly higher among nonsurvivors than survivors. Among subjects with indirect ARDS, mechanical ventilation-related parameters did not differ significantly between nonsurvivors and survivors, whereas serum lactate levels and pSOFA scores were significantly higher among nonsurvivors compared to survivors (Table 3).
Characteristics of Survivors and Nonsurvivors With Direct and Indirect Pediatric ARDS
In association with direct ARDS, but not with indirect ARDS, there was a significantly increasing trend of mortality with pediatric ARDS severity (Fig. 1). Among children with mild pediatric ARDS, the indirect subtype was associated with a higher mortality rate than the direct subtype (30.8% vs 0%).
Mortality according to pediatric ARDS severity. A: Direct pediatric ARDS and B: indirect pediatric ARDS. Dotted lines represent median mortality for each group (13.3% and 26.5% for the direct and indirect subtypes, respectively). Brackets with P values show individual comparisons of mortality between the 2 groups.
Discussion
Our results indicate that there are significant differences in the clinical features of the direct and indirect subtypes of pediatric ARDS, especially in comparisons of survivors with nonsurvivors. In terms of causes, this study included many more subjects with direct ARDS than with indirect ARDS. Pneumonia was the leading cause of the direct subtype, whereas sepsis was the most common initiating factor associated with indirect ARDS, which was consistent with previous reports.2,5,32,34,38
In this study, the overall mortality rate was 16%. This is comparable with previous studies in North America, Europe, and Australia-New Zealand, and much lower than what has been reported from other Asian countries.17,18,39 ARDS subtype (direct or indirect) was not found to predict mortality in our study. Findings regarding ARDS subtypes and mortality rates are inconsistent and frequently debated. Despite this, several studies of ARDS subtypes have reported similar mortality rates between the 2 subtypes, consistent with those observed in this study.27,32,34 However, to date, there have been no investigations of prognostic factors according to pediatric ARDS subtypes.
When mechanical ventilation-related variables were compared between survivors and nonsurvivors with the direct and indirect subtypes, for direct pediatric ARDS subjects,  , PEEP, peak inspiratory pressure,
, PEEP, peak inspiratory pressure, 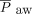 , and oxygenation index were significantly higher among nonsurvivors than survivors. Among these subjects, it was also observed that mortality and severity rose correspondingly.
, and oxygenation index were significantly higher among nonsurvivors than survivors. Among these subjects, it was also observed that mortality and severity rose correspondingly.
It has been reported that alveolar morphology, functional aspects, and responses to therapeutic interventions may differ according to ARDS subtype.29,40 In patients with direct ARDS, the alveolar epithelium is the first site of injury. This injury results in alveolar edema, which affects surfactant production and turnover, limits effective alveolar repair, and finally leads to fibrosis.41,42 This morphologic change results in more critically impaired oxygenation with higher 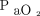 /
/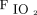 and lung injury scores, which is considered to be a surrogate for ARDS severity, compared with that which occurs in association with indirect ARDS.32,34 In terms of respiratory mechanics, direct ARDS was associated with higher physiologic dead space, higher lung static elastance, and lower chest wall elastance than indirect ARDS. This could influence the incidence of ventilator-induced lung injury and response to therapeutic interventions such as recruit maneuvers.32,43,44
and lung injury scores, which is considered to be a surrogate for ARDS severity, compared with that which occurs in association with indirect ARDS.32,34 In terms of respiratory mechanics, direct ARDS was associated with higher physiologic dead space, higher lung static elastance, and lower chest wall elastance than indirect ARDS. This could influence the incidence of ventilator-induced lung injury and response to therapeutic interventions such as recruit maneuvers.32,43,44
In patients with indirect ARDS, the primary lesion is diffuse damage of the vascular capillary endothelium. This is caused by the indirect, systemic insults of circulating mediators released from extrapulmonary foci into the blood.29,30 This can also result in further extrapulmonary organ dysfunction as well as respiratory failure. As a consequence, systemic factors rather than pulmonary or mechanical ventilation-related variables could play more of a role in the clinical course of indirect ARDS, including the associated mortality rate. Consistent with these findings, serum lactate levels and pSOFA scores in this study were markedly higher among nonsurvivors than survivors with indirect pediatric ARDS. Hyperlactatemia is a marker of tissue hypoxia and organ hypoperfusion, which could induce multiple organ dysfunction.45,46 In addition, extrapulmonary organ dysfunction commonly occurs concurrently with sepsis-induced respiratory dysfunction. Further, among patients with indirect ARDS, higher rates of organ failure have been reported to be a major risk factor for mortality.47 This finding was also consistent with our results. Notably, among subjects with the indirect subtype, the mortality rate was higher in association with mild cases than with moderate cases. The mortality rate among subjects with mild indirect ARDS was also higher than that among subjects with mild direct ARDS. This result is partially attributable to the fact that with the indirect subtype of pediatric ARDS, clinical outcomes, including mortality, had stronger associations with underlying causes and related systemic influences than with direct pulmonary injury.
It should also be noted that all patients with ARDS do not suffer from the same uniform disease, despite sharing the condition of hypoxemia. Therefore, the reversal of hypoxemia alone may be insufficient to improve clinical outcomes and reduce mortality among patients with ARDS. Based on the results of this study, various clinical factors could be associated differently with mortality in association with direct and indirect pediatric ARDS. Although there remains some controversy regarding the clinical relevance of pathophysiology in ARDS, the current results, in conjunction with the aforementioned findings, indicates that the cause of a lung injury and related subtypes may, therefore, affect the clinical course and outcomes in pediatric ARDS. Therefore, the classification of ARDS cases into subtypes by their distinct characteristics may serve to homogenize patient groups. This, in turn, would facilitate the identification of prognostic factors and the development of individualized treatment strategies for the improving clinical outcomes. However, further large-scale, multicenter studies are required to clarify the implications of this study’s findings.
While this study provided meaningful results, it did have some limitations. This was an exploratory, retrospective study with a small sample size from a single tertiary care center. Due to the characteristics of the institution, the causes of ARDS were not very diverse. None of the ARDS cases were caused by severe trauma, drowning, or inhalation injury. Additionally, there was a limited number of subjects with indirect pediatric ARDS who met the study criteria. These limitations in sampling may affect the generalizability of the findings. More extensive statistical analyses, including multivariable logistic regression and various subgroup analyses, could not be performed because of the limited size of the study sample and the low mortality rate. We attempted to include only cases in which ARDS was initiated by a single main cause. However, both direct and indirect injuries could have sequential or synergistic effects that result in some mixed pathophysiologic effects over the course of the disease, which could introduce some bias and lead to confounding elements influencing the study’s results. In this study, we only collected data on the day of pediatric ARDS diagnosis, which may limit time-dependent information associated with sequential effects.
Despite these limitations, this study had a number of advantages. It was a single-center study where unified diagnostic criteria were applied, and overall intensive and supportive management were consistent throughout the study period. To our knowledge, this is the first report to evaluate pediatric ARDS classified as direct and indirect subtypes, and we have reported remarkably distinct clinical features between surviving and nonsurviving ventilated, critically ill subjects with pediatric ARDS.
Conclusions
The direct and indirect subtypes of pediatric ARDS have different characteristics, especially between survivors and nonsurvivors. Although pediatric ARDS remains a devastating clinical problem with high mortality, our findings indicate that different causes and subsequent pathophysiologic factors associated with the direct and indirect subtypes play important roles in the clinical outcomes of pediatric ARDS. In the future, large-scale, multicenter, clinical investigations may provide a more thorough assessment of the differences between direct and indirect pediatric ARDS. This may help identify subtype-specific prognostic factors, which may lead to the development of evidence-based individualized therapeutic strategies to improve clinical outcomes.
Footnotes
- Correspondence: Won Kyoung Jhang MD PhD, Division of Pediatric Critical Care Medicine, Department of Pediatrics, Asan Medical Center Children’s Hospital, University of Ulsan College of Medicine, 88, Olympic-ro-43-gil, Songpa-gu, Seoul 05505, Korea. E-mail: wkjhang{at}amc.seoul.kr
The authors have disclosed no conflicts of interest.
- Copyright © 2020 by Daedalus Enterprises








