Abstract
BACKGROUND: Lung ultrasound is an examination that allows the assessment of pulmonary involvement by analyzing artifacts. Our primary aim was to correlate our lung ultrasound findings with pulmonary function and the modified Bhalla score in patients with cystic fibrosis.
METHODS: Subjects with cystic fibrosis were evaluated based on the results of lung ultrasound, pulmonary function exams (ie, spirometry before and after the use of a bronchodilator and SpO2), and the modified Bhalla score. The partial correlation set by age between lung ultrasound, pulmonary function, and modified Bhalla score was carried out. Lung ultrasound was graded according to a new score, ranging from 0 to 36, with a higher score being associated with a greater degree of involvement. We performed Bland-Altman and linear regression analysis to identify bias between lung ultrasound and modified Bhalla score. Alpha = 0.05.
RESULTS: 18 subjects with cystic fibrosis were included. In partial correlation controlled by age, we observed significant ultrasound score values with weight (partial correlation = −0.579), body mass index (partial correlation = −0.609), SpO2 (partial correlation = −0.728), FVC% (pre-bronchodilator: partial correlation = −0.538; post-bronchodilator: partial correlation = −0.560), FEV1% (pre-bronchodilator: partial correlation = −0.536; post-bronchodilator: partial correlation = −0.546), and modified Bhalla score (partial correlation = 0.607). We did not identify bias between lung ultrasound and modified Bhalla score measured by z-score.
CONCLUSIONS: Lung ultrasound seems to be effective and corroborates with high-resolution computed tomography when evaluated by the modified Bhalla score. At the same time, lung ultrasound had significant correlation with pulmonary function and nutritional status.
Introduction
Cystic fibrosis is caused by mutations in the CFTR gene (cystic fibrosis trasmembrane conductance regulator, 7q31.2).1 In cystic fibrosis, electrolyte imbalance affects lung secretions, which leads to chronic lung disease and is characterized by a cyclical process of inflammation and infection, culminating in bronchiectasis, progressive deterioration of the lung parenchyma, and progression to chronic respiratory failure, cor pulmonale, and often death.1–6 Several tools have been used to evaluate pulmonary function and structure and to determine the severity and progression of cystic fibrosis.7 Among the methods in use to evaluate the structure and evolution of lung deterioration in cystic fibrosis, there is high-resolution computed tomography (HRCT), which is considered the accepted standard option and is been recommended every 2 years throughout a patient's life.8 However, HRCT has numerous limitations, such as the use of radiation, restricting periodic reproduction of the exam, and anesthesia in infant and preschooler children, as well as the high cost of the procedure.9 In this context, there is a need to search for new methods to evaluate pulmonary damage in cystic fibrosis, such as lung ultrasound (see the supplementary materials at http://www.rcjournal.com).
We found no studies on the use of lung ultrasound to evaluate or compare lung deterioration in cystic fibrosis.10 However, it is known that the presence of air in the lungs and calcium in the bone structure hinder pulmonary evaluation and the use of ultrasound treatment for respiratory disease because it limits the reach of the ultrasound beam, producing images with artifacts.11 Technological advances in ultrasound transducer geometry, along with an understanding of artifacts that allows the evaluation of mediastinal and pleural lung lesions with acoustic anatomical windows (ie, supraclavicular, suprasternal, parasternal, and intercostal spaces) allow better evaluation of lung structures.12–14 Until now, the most widely used request for lung ultrasound is to detect pleural effusion.15
Lung ultrasound has several benefits. It is safe and free of any adverse effects, thus allowing serial evaluation. Lung ultrasound reduces radiation exposure and hospital cost (see the supplementary materials at http://www.rcjournal.com).16 In addition, lung ultrasound does not require sedation, and it is possible to conduct it while a patient is in bed. These characteristics have encouraged training and studies on the use of lung ultrasound in cystic fibrosis.
The primary objective of this study was to compare the effectiveness of lung ultrasound versus HRCT in evaluating pulmonary function in children, adolescents, and young adults with cystic fibrosis. Secondary objectives were to verify the description of the images of lung ultrasound considering the B-lines pattern, consolidation, pleural effusion, and recent findings; to verify the pairing of the findings of lung ultrasound with pulmonary function (ie, spirometry and SpO2); to verify the modified Bhalla score values and its association with lung ultrasound data; and to verify subject demographics and clinical data.
QUICK LOOK
Current knowledge
Among the methods available to evaluate the structure and evolution of lung deterioration in individuals with cystic fibrosis, high-resolution computed tomography (HRCT) has been considered the accepted standard, despite numerous limitations. There is a need to identify new methods to evaluate pulmonary damage in individuals with cystic fibrosis, such as lung ultrasound.
What this paper contributes to our knowledge
Lung ultrasound, which is a noninvasive and radiation-free procedure, is an effective method to evaluate pulmonary damage in subjects with cystic fibrosis, corroborating findings with HRCT and correlating significantly with pulmonary function and nutritional status.
Methods
Study Design
We conducted a cross-sectional non-randomized non-blinded study involving patients from a Brazilian cystic fibrosis reference center at the University of Campinas, Cidade Universitária Zeferino Vaz, São Paulo, Brazil. All subjects signed informed consent terms prior to study initiation. The study was carried out according to the Declaration of Helsinki. The study was approved by the ethics committee of the University of Campinas (64515817.4.0000.54.04). The study included subjects with 2 positive sweat chloride tests with values ≥ 60 mEq/L, monitoring at the reference center, and a HRCT exam within, or close to, the same time period as lung ultrasound for the purpose of data associations. Subjects who did not perform both exams (ie, lung ultrasound and HRCT) were excluded. We chose to include only subjects with cystic fibrosis who attended the out-patient clinic periodically (ie, ≥ 4 times/y) and had controlled digestive and pulmonary disease.
Chest HRCT was used as the reference test because it is the accepted standard for evaluating lung structures in patients with cystic fibrosis. Patients with cystic fibrosis undergo periodic tomographic evaluations, according to the routine of the referral service, which consists of chest HRCT every 2 years during periods of respiratory disease stability. All examinations were performed on the Multislice Aquillion 64-channel device (Canon Medical Systems, Tustin, California), with acquisition thickness of 1 mm, with parameters of 100 mA/s, from 100 kV to 120 kV. Tomographic evaluations were performed according to the protocol of the radiology department of the institution.
Cystic fibrosis is diagnosed in patients with compatible symptoms or a family history of cystic fibrosis after conducting 2 sweat tests with chloride values of ≥ 60 mEq/L in at least 2 samples or 2 pathogenic variants of the CFTR gene.17–19
For the study design, we adopted the PICO strategy: Population = subjects with cystic fibrosis who were monitored at our center with both HRCT and lung ultrasound; Intervention = lung ultrasound; Control = use of HRCT to validate lung ultrasound findings; and Outcome = to determine the effectiveness and correlation of lung ultrasound to HRCT and pulmonary function, and to assess associations with other demographic, clinical, and laboratory data.
Lung Ultrasound
Lung ultrasound was performed by the first author (AOP), who is a pediatric pulmonologist trained in point-of-care ultrasound. The findings were assessed by a second member of the team, who is a radiologist with experience in lung ultrasound. The images assessed by both researchers were then recorded. Researchers reached consensus about their findings.
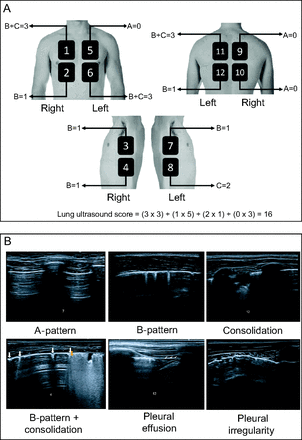
The areas indicated by international consensus20 on lung ultrasound were assessed, and the standards of pulmonary involvement, when present, were reported as A pattern, B pattern, consolidation, pleural effusion, and, if relevant, possible new findings (ie, pleural irregularities) (Fig. 1). The pulmonary involvement standard and ultrasound protocol have been described in the supplementary material (available at http://www.rcjournal.com).20–28
A: International consensus on lung ultrasound representing the areas assessed in the study: anterior superior, anterior basal, lateral superior, and lateral basal, plus zones delimited by parasternal and axillary lines (anterior and posterior); concomitantly, dorsal regions (posterior, superior, and basal) and those delimited by paravertebral and parascapular lines. The figure is courtesy of an author [THS]. B: Images of the main findings of the study, including B-pattern, consolidation, pleural effusion, and pleural irregularity.
According to the involvement standard, lung ultrasound was classified as interstitial syndrome: ≥ 2 regions with bilateral B-pattern involvement; lung consolidation: ≥ 1 region with consolidation involvement; combined: B-pattern and concomitant consolidation; normal: presence of A-pattern.
The degree of changes in the analyzed lung regions were numbered and classified with values ranging from 0 (minimum) to 3 (maximum), considering the presence or absence of B-pattern and consolidation. In the presence of B-pattern, the region scored 1 point; in the presence of consolidation, the region scored 2 points. With this format, the sum of both scores provided a maximum value of 3 points for each region. Because cystic fibrosis primarily involves the airways, the presence of consolidation needed to result in a higher number of points. In the analysis, the lung was divided into 12 anatomical regions, which allowed for a maximum score of 36 (Fig. 1). We did not score the presence of pleural effusion because it was very rare in this study. The presence of pleural irregularities was only used when describing the findings. All subjects had their lung ultrasound findings compared to HRCT.
Markers Used
The following cystic fibrosis severity markers were used in the study: sex and age (in years); CFTR mutations; modified Bhalla score (see the supplementary materials at http://www.rcjournal.com), calculated per the work of Folescu et al29 (ie, “the total score for each patient was obtained by summing the scores for each morphological change, which were attributed on the basis of the severity/extent of the abnormality … [and] the total score can range from zero (absence of abnormalities) to 36 (all abnormalities present and severe)”; body mass index (kg/m2); pancreatic failure measured by fecal elastase levels; and markers of pulmonary condition (ie, spirometry, SpO2, and isolated microorganisms associated with cystic fibrosis). A survey of the lung microbiota was carried out for the microorganisms mainly associated with cystic fibrosis in our center: Staphylococcus aureus, Pseudomonas aeruginosa (mucoid and non-mucoid), Achromobacter xylosoxidans, Burkholderia cepacia, and Stenotrophomonas maltophilia.
Pulmonary Function
Spirometry was carried out with the use of a spirometer (CPFS/D, MedGraphics, Saint Paul, Minnesota). Data were recorded using Breeze PF 3.8 (Breeze Software, Dallas, Texas), according to the reference literature.30,31 The following parameters were evaluated: % of predicted FVC, % of predicted FEV1%, relationship between FEV1 and FVC, and FEF25–75% (see the supplementary materials at http://www.rcjournal.com).
Statistical Analysis
Statistical analysis was conducted with SPSS (IBM, Armonk, New York) and MedCalc Statistical Software version 16.4.3 (MedCalc Software bvba, Ostend, Belgium). In all analyses, alpha = 0.05 and a 2-tailed approach were considered. All participant data were obtained in this study; no compensation for missing data was necessary.
A descriptive analysis was performed with categorical data presented as absolute and relative frequency, and numerical data are presented as mean ± SD; median and range; and 95% CIs for the average. The normality of the numerical data were evaluated with analysis of descriptive measures for central tendency, a graphical method (normal Q-Q plot, Q-Q plot without trend and boxplot), and Kolmorov-Smirnov and Shapiro-Wilk normality tests.
The association between categorical data were carried out with the Fisher exact test, and when P < .05, data were described with the odds ratio estimate and the conditional maximum-likelihood estimate (cMLE) presentation. The association between the numbered data of independent groups was conducted with the Mann-Whitney U test of independent samples, with data being presented as median and range.
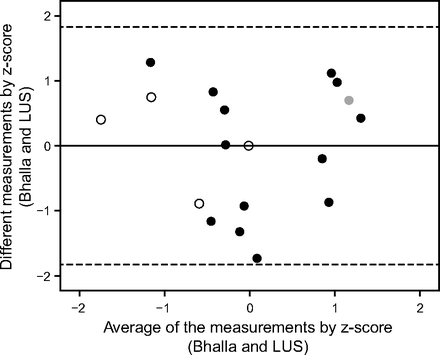
Bhalla score, spirometry, SpO2, B-pattern, lung ultrasound score, and body mass index were compared using partial correlation controlled by age. Additionally, we performed the analysis of linear regression between the different measurements (y axis = [measurement 1 − measurement 2]) and the measurement average (x axis = [measurement 1 + measurement 2]/2) for bias analysis by comparing the differences between the data in the Bland-Altman plot, indicating the obtained values in the lung ultrasound score and Bhalla score.
Data from Bhalla scores were called measurement 1, and data from lung ultrasound were called measurement 2. To compare the data, we opted to use the z-score, which allowed testing with the same scale in both measures calculated as [(absolute value − average)/standard deviation]. A Bland-Altman plot was used to analyze the agreement between the lung ultrasound and modified Bhalla scores, and this technique was also used with linear regression to assess dose-dependent bias (Fig. 2). We also used the z-score to promote the feasibility of running the test using the same scale for the results of both examinations. The horizontal lines were drawn at the mean difference and limits of agreement, which were defined as the mean difference ± 1.96 × the standard deviation of the differences. In addition, we used linear regression to assess dose-dependent bias on a Bland-Altman plot.
Bland-Altman plot to verify the presence of bias between modified Bhalla and lung ultrasound score (LUS) evaluated by z-score. We calculated the z-score with the formula z = (absolute value − mean)/SD. Linear regression analysis returned R = 0.001, R2 < 0.001, R2 adjusted = 0.61, P = .99. Gray circles = presence of pleural effusion. Black circles = B-pattern plus consolidation (joint). Empty circles = B-pattern (interstitial syndrome). Center line shows the bias, and dashed lines represent the upper and lower limits of agreement.
Results
We performed lung ultrasound scans in 18 subjects. Table 1 details the clinical, demographic and laboratory data of all subjects. Most subjects were predominantly colonized by S. aureus (14 of 18, 77.8%) and by P. aeruginosa (8 of 18, 44.4%). At the same time, we observed that 14 of 18 (77.8%) subjects showed pancreatic failure, and the same percentage showed pulmonary involvement presenting with altered value of FEV1 (%). In the genetic analysis, we observed a high prevalence (22 of 36, 61.1%) of the p.Phe508del allele. In addition, the modified Bhalla score ranged from 6 to 30 points, with a mean of 22 points.
Clinical Characteristics, Pulmonary Function, Modified Bhalla Score, and Lung Ultrasound Data of Subjects With Cystic Fibrosis
For lung ultrasound, we classified subjects into the following categories: combined involvement: 13 of 18 (72.2%); combined involvement + pleural effusion: 1 of 18 (5.6%); and interstitial syndrome: 4 of 18 (22.2%) (Table 1). All subjects presented with the B-pattern, which ranged from 5 of 12 to 12 of 12 affected lung regions. However, consolidation occurred in 3 lung zones of 5 subjects; 4 subjects showed no consolidation. The lung ultrasound scores ranged between 6 and 18 out of a total of 36 (Table 2).
Main Findings in Lung Ultrasound in Subjects With Cystic Fibrosis Describing the Assessed Regions of the Lung and the Proportion Between B-Pattern and Consolidation for the Categorization of Pulmonary Involvement
There were no significant associations between the lung ultrasound score and B-pattern with categorical data (ie, bacteria or comorbidities). In addition, we compared lung ultrasound classification of combined versus interstitial with clinical, demographic, and laboratory markers, noting greater severity in the combined pattern for:
FEV1 (%) < 80% (P = .02), estimated odds ratio = 25.76 (cMLE 95% CI for Fisher exact test = 1.151–2,194.00) for combined pattern (13 of 14) versus interstitial syndrome (1 of 14);
SpO2 (P = .01) → combined pattern: 95.50 (88–97), interstitial syndrome: 97.50 (96–99);
post inhaled bronchodilator (BD) FEV1 (%) (P = .035) → combined pattern: 53.50 (25–106), interstitial syndrome: 80.50 (57–102);
post-BD FEF25–75% (P = .046) → combined pattern: 28.50 (15–122), interstitial syndrome: 79.50 (29–89);
response to BD for FEV1 (%) (P = .046) → combined pattern: 0.5 ([minus10 to 11), interstitial syndrome: 3.5 (2–7);
modified Bhalla Score (P = .046) → combined pattern: 23.50 (7–30), interstitial syndrome: 14 (6–20).
Table 3 shows the individual data of subjects regarding spirometry, democraphic, and clinical data. Table 4 presents the partial correlation for age between the lung ultrasound score and the anthropometric and pulmonary function data (ie, SpO2 and spirometry). In this correlation, there was an association between the functional and structural values, respectively, measured with spirometry + SpO2 and the modified Bhalla score. The B-pattern showed partial correlation controlled by age: weight: −0.610 (P < .01); body mass index: −0.610 (P < .01); and SpO2: −0.559 (P = .02) (Table 4). Using the total score, there was also partial correlation controlled by age: weight: −0.579 (P = .02); body mass index: −0.609 (P < .01); SpO2:−0.728 (P < .01); pre-BD FVC (%): −0.538 (P =.03);post-BD FVC (%): −0.560 (P = .02); pre-BD FEV1 (%): −0.536 (P =.03); post-BD FEV1 (%): −0.546 (P = .02); and modified Bhalla score: 0.610 (P = .01) (Table 4). Table 4 also shows the partial correlation controlled by age between the modified Bhalla score and spirometry data: post-BD FVC (%): −0.551 (P = .02); pre-BD FEV1 (%): −0.490 (P = .046); and post-BD FEV1 (%):−0.504 P = .039).
Main Clinical and Laboratory Findings of Subjects With Cystic Fibrosis
Partial Correlation Controlled by Age Between Lung Ultrasound Score, B-Pattern,* Anthropometric Data, and Pulmonary Function in Subjects With Cystic Fibrosis
Discussion
In our study, there was a correlation between lung ultrasound data with the structural and functional evaluation of the lung indicated by the modified Bhalla score and with pulmonary function (spirometry and SpO2). All subjects showed pulmonary involvement characterized by B-pattern. We also identified consolidation, although in a smaller proportion. To our knowledge, this is the first study to describe the correlation between lung ultrasound and structural and functional changes in children and adolescents with cystic fibrosis.
The HRCT is considered the accepted standard test for evaluating pulmonary structural involvement in cystic fibrosis.8,10 However, the ionized radiation exposure at the HRCT exam is the major limitation to its use. Thus, there is a need to develop and validate new tools that can evaluate structural abnormalities and that can be carried out concurrently with pulmonary function analysis. Within this context, we highlight the use of lung ultrasound as a viable way to monitor and follow up lung disease in cystic fibrosis, given that this tool has been validated for other diseases.20,24,32
A notable feature of lung ultrasound is its utility in evaluating changes in the pleura and its surrounding areas, in which it is possible to note interstitial abnormalities at an early stage.33 In our study, the presence of alterations in the lung parenchyma was evident because it was closely associated with the B-pattern in lung ultrasound, which is a symptom of diffuse parenchymal lung disease.34 However, understanding the ultrasound scan and the B-pattern as a parameter still requires investigation.
In our data, lung ultrasound indicated interstitial lung disease in all subjects. This suggests alterations in the pleural line, which can include irregularities, fragmentation, thickening, subpleural abnormalities, and the heterogeneous distribution of the B-lines. Among these signals, we can characterize the cystic fibrosis phenotype, in which we observed inflammatory disease with predominance of the B-pattern. Of note is the importance of monitoring inflammation, which is related to decreased lung function and a higher risk of bronchiectasis, and in most cases it is directly associated with chronic lung infection. In this context, lung ultrasound is a tool that could be used to evaluate inflammation mainly demonstrated by B-lines and other artifacts of pleural irregularities. In addition, when evaluating interstitial commitment in cystic fibrosis, we found greater visibility (ie, sensitivity and specificity) using lung ultrasound compared to the HRCT.
HRCT is indicated every 2 years, and it is initiated on the basis of varying clinical and laboratory criteria, although in many cases structural changes occur prior to functional alterations. Many patients with cystic fibrosis present with clinical changes, such as chronic cough, hypersecretion, mucus thickening, lung auscultation alterations, and structural changes, in the presence of normal or minimally altered pulmonary function.
Lung ultrasound allows for frequent patient evaluation, and the procedure can be performed during all consults or interventions, which allows for the diagnosis and follow-up of the progressive and chronic nature of cystic fibrosis using various ultrasound markers. Contributing to this need is the increased life expectancy of individuals with cystic fibrosis, which is associated with more cystic fibrosis–related comorbidities, as well as comorbidities that are not associated with the disease.35 We predict an increase in the number of patients with other diseases, including cancer, in the future, therefore we should avoid the use of procedures that might serve as triggers for other diseases, such as the use of radiation in HRCT.
The literature clearly outlines the high correlation between spirometry and lung structure markers obtained with radiography and HRCT, especially in severe lung disease.36–39 However, the use of lung ultrasound can enable a better understanding of the intrinsic association between function and structure, considering that, in our findings, we observed a significant correlation between the results of lung ultrasound, modified Bhalla score, spirometry, SpO2, and body mass index.
The applicability of lung ultrasound extends to the identification of areas with high secretion accumulation, which may be addressed by the health care team. We believe that lung ultrasound results might be used as a new biomarker in clinical studies, mainly regarding the investigation of treatment response to new antibiotics or anti-inflammatory drugs in patients experiencing an exacerbation. The use of lung ultrasound in clinical research is of high clinical importance, it is low cost, it is easy to implement and interpret, and it is associated with functional and structural damage, the absence of collateral effects, and high reproducibility. In addition, lung ultrasound can be conducted serially, with numerical and categorical analyses, which corroborates with the intense search for new markers.
In summary, we believe that lung ultrasound is feasible and can provide evidence of artifacts associated with the presence and severity of lung disease in patients with cystic fibrosis. However, artifact interpretation requires further investigation among researchers to understand whether an artifact represents the presence of interstitial involvement, as shown in other lung diseases using the same imaging technique. We also realize that interstitial involvement is not widely discussed as a pathologic feature in cystic fibrosis, and thus our findings may run counter to this theory. In support of our findings, we observed some HRCT images with evidence of early-stage intralobular septal thickening as evaluated by the modified Bhalla score, which represents a feature of interstitial lung involvement.
Our study had two primary limitations. We evaluated a small and heterogeneous sample, and our statistical analysis was exploratory in nature due to the low power of the sample, raising the risk of type-2 error. As strengths, we have noted that lung ultrasound is a noninvasive and radiation-free exam, which is important information for the management of cystic fibrosis. In addition, there currently is no consensus on when to conduct the first HRCT, so early identification of interstitial involvement on lung ultrasound could indicate the best time to perform an initial HRCT. Furthermore, lung ultrasound can improve the care and monitoring of patients with cystic fibrosis. Finally, despite being an operator-dependent technique, short-term training on lung ultrasound could contribute to a better understanding of the tool.
Conclusions
In this study, lung ultrasound appeared to have concordance with HRCT when evaluated using the modified Bhalla score. In our results lung ultrasound had a significant correlation with pulmonary function and nutritional status. In addition, lung ultrasound was feasible for identifying the B-pattern in all participants, and we were able to determine the presence of consolidation, combined involvement (ie, B-pattern + consolidation), pleural effusion, and other pleural irregularities. Further studies should be conducted to validate and expand our findings.
Footnotes
- Correspondence: Andressa O Peixoto MD MSc, Department of Pediatrics, School of Medical Sciences, University of Campinas, Tessália Vieira de Camargo, 126, Cidade Universitária Zeferino Vaz, Campinas, São Paulo, Brazil, CEP 13083-887 or Fernando AL Marson PhD, Laboratory of Medical and Human Genetics, Post Graduate Program in Health Science, São Francisco University, Avenida São Francisco de Assis, 218, Jardim São José, Bragança Paulista, São Paulo, Brazil, 12916-900. E-mail: andressa_op{at}hotmail.com or fernando.marson{at}usf.edu.br.
Dr Peixoto is partially supported by the Brazilian National Council for Scientific and Technological Development (#407364/2016-1). Dr Marson is partially supported by the São Paulo Research Foundation (#2011/12939-4, #2011/18845-1, #2015/12183-8, and #2015/12858-5), and the University of Campinas Fund for the Support of Education, Research and Extension (#0648/2015). Dr Ribeiro is partially supported by the São Paulo Research Foundation (#2011/18845-1, #2015/12183-8) and the Brazilian National Council for Scientific and Technological Development (#407364/2016-1). The authors have disclosed no conflicts of interest.
Supplementary material related to this paper is available at http://www.rcjournal.com.
- Copyright © 2020 by Daedalus Enterprises