Abstract
BACKGROUND: Air-flow oscillations generated by exhaling through oscillatory positive expiratory pressure (OPEP) devices favor airway clearance. Variations in mechanical properties between different devices may influence therapeutic efficacy. The objective of this study was to assess mechanical properties in vitro and to compare the performance of 6 OPEP devices at different resistance levels under active expiratory flow patterns.
METHODS: 4 gravity-dependent OPEP devices (ie, Flutter, Gelomuc, Pari O-PEP, Shaker Medic Plus) and 2 gravity-independent OPEP devices (ie, Acapella Choice and Aerobika) were each tested at low, medium, and high resistance settings. All devices were independently connected to a pulmonary waveform generator that reproduced active exhalation flows. Expiratory flow-volume curves were retrieved from 4 subjects with different stages of obstruction severity and were scaled according to either peak expiratory flow (4, 6, and 8 L/s) or volumes (2, 3 and 4 L), thus amounting to 24 active exhalations. Resulting waveforms were divided into 4 parts and the 2 middle parts were used to extract the following mechanical data: positive expiratory pressure (PEP), maximum expiratory pressure (Ppeak), oscillation frequency, and flow oscillation amplitude. The percentage of tests achieving oscillation frequencies ≥ 12 Hz and PEP ≥ 10 cm H2O was calculated for each device.
RESULTS: Mechanistic effects of the Acapella, Aerobika, and Shaker devices were not comparable. The Flutter, Gelomuc, and Pari devices behaved similarly and achieved more tests with optimum oscillation frequency and PEP values than the other devices. These 3 devices also produced the highest oscillation amplitudes at the low-resistance level, whereas the Aerobika elicited higher and consistent oscillation amplitudes at medium and high resistance settings.
CONCLUSIONS: Operational parameters differed between and within devices, yet the Flutter, Gelomuc, and Pari devices were similar in many aspects. Therapeutic efficacy may depend on the selected OPEP device and set resistance.
- physiotherapy
- airway clearance techniques
- oscillatory positive expiratory pressure
- OPEP
- secretion clearance
- air flow oscillations
- mechanical evaluation
Introduction
Airway clearance techniques account for a substantial part of the chest physiotherapy treatment arsenal in patients with chronic suppurative lung diseases, such as cystic fibrosis or non-cystic fibrosis bronchiectasis.1 By speeding up the elimination of excessive phlegm obstructing the lung, these techniques are used to limit the occurrence of recurrent pulmonary exacerbations and to stabilize the decline of lung function.
To assist airway clearance, oscillatory positive expiratory pressure (OPEP) devices are frequently applied in clinical practice, and numerous research studies have investigated their functioning and efficacy.2,3 Basic principles of OPEP involve an expiratory oscillating air-flow brake, thereby inducing 2 main physiological effects. First, positive expiratory pressure (PEP) is generated and stabilizes the airways by means of a pneumatic splint to prevent early airway collapse during expiratory efforts.4 Second, air-flow oscillations attempt to stimulate ciliary beat frequency and coincide with the respiratory system resonance frequency. Moreover, turbulent air-flow spikes that are elicited reduce mucus viscoelasticity and promote its detachment and cephalad movement. Hence, air-flow oscillations facilitate mucociliary clearance.5–8
However, clinical studies comparing the efficacy of various OPEP devices have failed to report the superiority of any one technique over another.3 Beyond methodological considerations, these findings may partly result from mechanistic differences between OPEP devices. Theoretically, optimized OPEP utilization should generate oscillations of ≥ 12 Hz9,10 while reaching a minimum PEP of 10 cm H2O11 to achieve the above-mentioned physiological effects. Because many devices are manufactured with specific technology to produce air-flow oscillations, it is unclear whether the desired physiological effects are achieved when a subject exhales through a particular device adjusted at a given resistance level. Indeed, their mechanical effects such as PEP, oscillation frequency, and oscillation amplitude are strongly dependent on how the devices are used (eg, position, resistance settings, expiratory flow).10,12–14 In addition, between-device differences may interact with varying expired air-flow patterns obtained from patients with diverse conditions or disease severity.15 In these conditions, suboptimum OPEP utilization is likely, which may, in turn, affect therapeutic efficacy.
In vitro studies measuring and comparing mechanical effects of different OPEP devices by changing resistance settings, expiratory flows, and expiratory waveforms may help improve their use. Therefore, the purpose of this study was to characterize the mechanistic properties of 6 OPEP devices at different resistance settings and submitted to various expiratory flow curve patterns. We hypothesized that performance characteristics (ie, the likelihood of achieving oscillation frequency ≥ 12 Hz and PEP ≥ 10 cm H2O) differ between devices. We focused on commercially available, demountable, and easy to disinfect OPEP devices that are suitable for use in patients with chronic respiratory diseases.
QUICK LOOK
Current knowledge
Oscillatory positive expiratory pressure (OPEP) devices are frequently used to assist airway clearance. Because the underlying technology to produce airflow oscillations differs between existing devices, their mechanical behavior is assessed in vitro. Previous laboratory studies have generally assessed performance characteristics of OPEP devices under a range of constant flows, which may not reflect their performance in clinical use.
What this paper contributes to our knowledge
This simulation study used a range of active airflow exhalations to challenge the mechanical properties of 6 OPEP devices. Relevant similarities and differences in performance characteristics between devices have been recorded. Our findings provide guidance for clinicians to select and tune a device according to the intended therapeutic purpose.
Methods
OPEP Devices
We tested 4 gravity-dependent OPEP devices: Flutter VRP1 (Scandipharm, Birmingham, Alabama), Shaker Medic Plus (NCS Indústria e Comércio de Aparelhos Hospitalares LTDA, Baruerí, São Paulo, Brazil), Pari O-PEP (PARI Medical, Surrey, United Kingdom), and Gelomuc (G. Pohl-Boskamp GmbH, Hohenlockstedt, Germany) and 2 gravity-independent OPEP devices: Aerobika (Trudell Medical International, London, Ontario, Canada) and Acapella Choice (Smith's Medical, Ashford, Kent, United Kingdom). This study was performed at Cliniques universitaires Saint-Luc, Brussels, Belgium.
The Flutter, Gelomuc, Pari, and Shaker devices have a pipe-like design and behave similarly. They all have a removable perforated cap with a steel ball inside that is positioned on a conical cavity. During exhalation through the instruments, the steel ball vibrates while braking the expiratory flow, thereby provoking air-flow oscillations and PEP. The angle at which the device is held affects the amplitude and frequency of oscillations.13
The Acapella consists of a magnet, a counterweighted lever, and a cone on the end of the lever occluding the expiratory air flow. When exhaling through the device, the pressure increases until the expiring air pushes the cone up. The magnet brings the lever with the cone back to its original position, generating intermittent expiratory occlusion and air-flow oscillations. PEP levels and the amplitude and frequency of the vibrations are adjusted by rotating a knob at the distal end of the apparatus, which modifies the proximity of the magnet with the counterweighted lever.16
The Aerobika device is divided into 3 parts: a top case, a bottom case, and a valve cartridge. The one-way valve chatters during exhalation, creating intermittent resistance to exhalation and air-flow oscillations. A dial located in the front of the bottom case is used to adjust the resistance of the one-way valve.17
Each device was tested at 3 resistance levels. For the Flutter, Pari, and Gelomuc, low, medium, and high resistance settings corresponded respectively to an angle of 0°, 15°, and 30° between the horizontal line and the device's tube. Because the tube of Shaker Medic Plus was incurvated, the angulation of the conical cavity inside the device was adjusted to match the position of other gravity-dependent devices. For the Acapella and Aerobika devices, the dial or knob was set to the minimum, intermediate, or maximum position to represent low, medium, and high resistance levels, respectively.
Experimental Setup
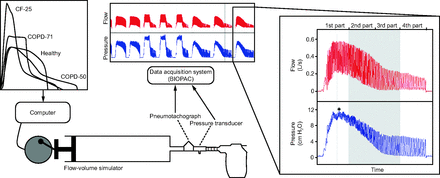
Each device was independently connected to a pulmonary waveform generator (Hans Rudolph flow-volume simulator, Shawnee, Kansas) to reproduce active expiratory flow patterns (Fig. 1). The pulmonary waveform generator is a computerized piston pump driven by a servo motor and is used in the validation of spirometers. Expiratory air-flow curves were collected among adult subjects exhaling through their own OPEP device according to their physiotherapist's standardized instruction (recording for research purposes was approved by the local ethics committee - 2016/08AOU/358). Typical instructions were to exhale actively but not forcefully, to achieve exhalation durations 3–4 times the duration of the preceding inhalation and to try to sustain a constant expiratory flow over this period. Replicate exhalation patterns (no. = 10) were recorded, and the averaged waveform profile of each subject was used (Fig. 1). For this study, we registered the expiratory flow curves of 1 healthy volunteer with a predicted value of FEV1 (FEV1%) of 103%, 1 subject with COPD and FEV1% of 71%, 1 subject with COPD and FEV1% of 50%, and 1 subject with cystic fibrosis and FEV1% of 25%. Time, pressure, flow, and integrated volume signals were recorded at a sampling rate of 500 Hz (MP150 System and AcqKnowledge software, BIOPAC Systems, Santa Barbara, California). Data were stored in a spreadsheet and smoothed and scaled using Matlab R2014a software (MathWorks, Natick, Massachusetts) so that the peak expiratory flow of each subject achieved 4, 6, and 8 L/s or the expiratory volume achieved 2, 3, and 4 L. Therefore, 24 active exhalations (4 waveforms × 6 scaled flow-volume exhalations) were uploaded on the flow-volume simulator and were injected into the 6 OPEP devices (Fig. 1).
Experimental setup when the flow-volume simulator was connected to the Aerobika device at high-resistance setting. Four different waveforms, obtained among subjects with different obstruction severity, were uploaded on the flow-volume simulator. The inset presents the third repetition of the waveform originating from the subject (COPD-50), which has been scaled so that peak expiratory flow achieved 0.4 L/s. The gray area shows the middle 2 parts of the expiration phase that were analyzed to compute mean positive expiratory pressure, flow oscillation amplitude, and oscillation frequency. Asterisk indicates maximum pressure measured on this expiration.
Data Collection and Performance Characteristics
The scaled active exhalations were injected into the OPEP devices. For each scaled active exhalation, measurements were organized in a run consisting of 3 repetitions, and the mean of the threefold repetition was used for statistical analysis. The exhalation phases produced were divided into 4 equal time parts, and the mechanical variables of mean PEP, oscillation amplitude, and oscillation frequency generated from the middle (second and third parts) were extracted and analyzed (Fig. 1). The maximum pressure (Ppeak) reached was also registered over the entire expiration phase. Mean PEP is the mean pressure generated, Ppeak is the highest pressure registered, oscillation frequency is the number of oscillations per second, and oscillation amplitude is the mean difference between lower and higher (peak-to-peak) flow values. Data were acquired at a sampling rate of 500 Hz via a pneumotachograph and a pressure transducer connected to the MP150 BIOPAC station calibrated beforehand, and data were further stored in a computer for subsequent analysis with AcqKnowledge software. Mean PEP and Ppeak were obtained directly. The oscillation amplitude was measured with the Matlab findpeaks function, a peak being considered as every point greater than its two neighbors. The amplitude of each oscillation was defined as the ordinate difference of the two following peaks. The oscillation frequency was obtained by means of fast Fourier transform. As the spectrum could be spread, the maximum amplitude of fast Fourier transform bin could not accurately reflect the dominant oscillation. The estimate of frequency was then performed by finding the maximum-area 5 Hz interval. This was done by testing all intervals.
Air-flow curves generating an oscillation frequency ≥ 12 Hz with a mean PEP ≥ 10 cm H2O during the second and third parts of the exhalation phase were considered optimum tests. According to these predefined criteria, performance was defined as the proportion of tests (ie, active exhalations) achieving both oscillation frequency and PEP targets and was calculated for each device operating at low, medium, and high resistance settings. Because the literature does not provide theoretical optimum oscillation amplitude values, the latter were not included in the definition of performance. However, devices and resistance settings that produced the greatest oscillation amplitude values were highlighted because air-flow oscillations with high peak-to-peak spikes are conceivably best suited to enhance airway clearance.
Statistical Analysis
Repeated measures analysis of variance with effects for 6 devices, 3 resistance levels, and 4 waveforms along with their interactions was used to investigate variations in PEP, Ppeak, oscillation frequency, and oscillation amplitude. Devices and resistance levels were considered as intra-subject variables, whereas waveforms were considered as an inter-subject factor. Whenever interactions between waveforms and devices or resistance levels were noticed, mechanical data were examined for each type of waveform individually. Categorical data were compared using the chi-square test. Analyses were performed with SPSS version 25 software (IBM, Armonk, New York). All statistical tests were 2-tailed. A P value of ≤ .05 was considered to be statistically significant.
Results
Statistically significant main effects for device and resistance along with their interactions were detected for each variable of interest (Table 1). Main effects indicate that both devices and resistance settings affected all mechanical parameters. Interactions describe that mechanical outcomes variations induced by a resistance step change were not interchangeable from one OPEP device to another. Significant interactions with waveforms were also found for oscillation frequency only, indicating that the different waveforms also contributed to oscillation frequency characteristics (Table 1).
Results (P Values) of Repeated Measures Analysis of Variance for the Effect of Device, Resistance Level, and Waveforms Along With Interactions
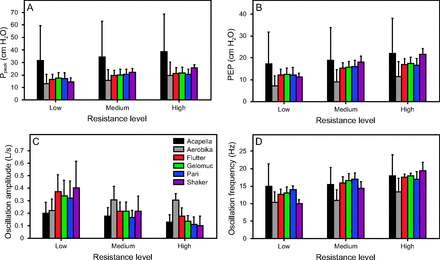
Mean values of mechanical variables are displayed in Table 2 and Figure 2. Increasing resistance consistently increased mean PEP, Ppeak, and oscillation frequency in all devices. Conversely, increasing the resistance level decreased oscillation amplitude in all devices with the exception of the Aerobika, where oscillation amplitude increased from low to medium resistance and then remained stable at high resistance setting. Switching the resistance settings of the Flutter, the Gelomuc or the Pari device had similar influence on mechanical variables, whereas the magnitude of change was greater with the Shaker device (Fig. 2). Compared to other OPEP devices, the Aerobika produced lower PEP, Ppeak, and oscillation frequency values at all resistance settings but higher oscillation amplitude values at medium and high resistance levels. The Acapella produced the highest mean PEP and Ppeak values for each resistance setting, and the highest oscillation frequency for the low resistance level. At medium and high resistance settings, the Acapella and the gravity-dependent devices produced oscillation frequency values that were in a similar range.
Variables of Interest by Device and Resistance Level
Operational parameters outputs by device and resistance setting. Mean (SD) values for (A) maximum pressure (Ppeak), (B) positive expiratory pressure (PEP), (C) flow oscillation amplitude, and (D) oscillation frequency for the different devices and resistance settings.
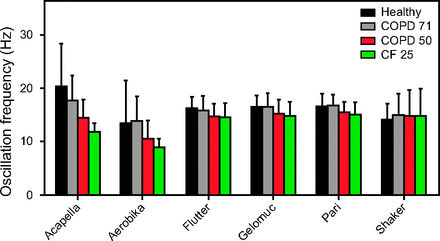
Figure 3 shows that waveforms had little impact on oscillation frequency in all gravity-dependent devices. This impact was more prominent in gravity-independent devices (mean oscillation frequency changes reached up to 8.5 Hz and 4.6 Hz for the Acapella and Aerobika devices, respectively). These results indicate that oscillation frequency generated by gravity-independent OPEP devices are inconsistent between different waveform profiles.
Mean (SD) values for oscillation frequency for the different devices and waveforms. COPD-71 = subject with COPD and FEV1% of 71%; COPD-50 = subject with COPD and FEV1% of 50%; CF-25 = subject with cystic fibrosis and FEV1% of 25%.
Performance characteristics (ie, the proportion of active exhalations reaching theoretical optimum oscillation frequency and PEP values per device and per resistance level) are presented in Table 3. Increasing the resistance level consistently improved the performance of each device. All gravity-dependent devices had excellent performance characteristics at medium and high resistance settings. Those devices were found to be more likely to produce optimum tests compared to the Acapella or Aerobika devices. At low resistance settings, the Shaker device produced a lower number of successful tests than the other gravity-dependent devices. Differences in performance between OPEP devices were highly significant (P < .001) when considering all resistance levels together or the low resistance setting only. Statistical analyses were precluded at medium and high resistance settings due to violation of assumptions to conduct the chi-square test, but visual inspection indicated findings similar to those for low resistance settings.
Performance Characteristics Expressed as the Percentage of Tests Achieving Optimum Oscillation Frequency and PEP Values
Discussion
This in vitro study determined and compared the mechanical characteristics and performances of 6 commonly used OPEP devices under expired air-flow curves that were retrieved among subjects with different lung disease severity. The devices were selected on the basis of their ability to be properly disinfected, which is a requirement for devices used with subjects susceptible to lung infections, such as patients with cystic fibrosis. Our results indicate that the Flutter, Gelomuc, and Pari OPEP devices behaved very similarly under different conditions and produced optimum mechanical variables to assist airway clearance, especially at medium and high resistance levels. In contrast, the mechanistic parameters of the Acapella, Aerobika, and Shaker devices were not comparable. Not only they did not respond similarly to different operational conditions, they were also less prone to produce oscillation frequencies ≥ 12 Hz while maintaining a PEP value of ≥ 10 cm H2O. However, the Aerobika produced higher oscillation amplitude values at medium and high resistance settings.
To our knowledge, this is the first study that used real active exhalations to challenge the mechanical properties of OPEP devices. Indeed, expiratory air-flow curves collected among adult subjects while they exhaled through an OPEP device have been further scaled so that they represented several clinical conditions. Previously, Van Fleet et al12 characterized mechanical properties of 4 OPEP devices by using a simulated lung model to mimic a symmetrical active exhalation from a child with cystic fibrosis and with moderate to severe lung disease severity. Other studies have evaluated the performance characteristics of one or several OPEP devices obtained across constant air flows.10,13,14,16,18–21 However, in clinical practice the expiratory air flow of patients exhaling through OPEP devices is hardly measured and scarcely constant. Simulation studies are therefore essential to optimize the use of the OPEP technique.
We described clinically relevant similarities and differences in performance characteristics of devices and their ideal settings to favor airway clearance. Although appropriate PEP values described are those ranging between 10 and 20 cm H2O,22 higher PEP levels are at least as effective to produce physiological meaningful effects11 as long as they do not induce excessive respiratory muscle fatigue.23 The optimal PEP value was then determined as ≥ 10 cm H2O.
Optimum oscillation frequency values are more complex to define, depending on whether the goal is to approximate the natural frequency of the ciliary beat, to coincide with the respiratory system resonance, or to reduce mucus viscoelasticity. Studies carried out in humans suggest that the natural cilia beating frequency is 11–15 Hz in the nasal cavity or the tracheobronchial tree.24–26 On the other hand, the resonance frequency of the respiratory system is slightly higher in healthy participants, with a wider range of normal values, depending on age, sex, and height, among other things.27,28 Furthermore, studies have shown that the respiratory system resonance frequency is increased in subjects with respiratory diseases and depending on the severity of airway obstruction.29,30 Our findings indicate that the Aerobika and Acapella devices generated lower oscillation frequency values under simulated expiratory waveforms representing significant air-flow obstruction, so their therapeutic efficacy may be mitigated in patients with more advanced lung disease severity. However, this extrapolation must be made with caution because we only tested 4 different expiratory waveforms. Regarding mucus rheology, it is known that air-flow oscillation frequencies of 22 Hz have a greater impact on the viscoelasticity of mucus gel stimulants than oscillatory frequencies around 12 Hz.31 In addition, App et al5 demonstrated that oscillation frequencies of 19 Hz with the Flutter device reduced sputum elasticity of cystic fibrosis mucus. Altogether and in agreement with other authors, we arbitrarily chose a minimum oscillation frequency value of 12 Hz to describe optimal OPEP mechanical performance.13,18
Ideal oscillation amplitude levels have not been characterized in the literature. However, the amplitude of air-flow spikes likely interacts with oscillation frequency to break down macromolecular interactions binding the sputa. Therefore, it is reasonable to presume that the higher the amplitude, the greater the bursts of expiratory air flow and the greater the reduction in mucus viscoelasticity. When reported, laboratory studies preferentially focus on pressure-oscillation amplitude rather than flow-oscillation amplitude, in contrast to our study.12,14,16,20,21 However, these parameters are not transposable because the relation between them is far from being evident, just as it is graphically presented in Figure 1. In our study we displayed flow-oscillation amplitude because it is actually short bursts of increased expiratory air flow that help to mobilize lungs secretions, as with coughing.32 Moreover, pressure values are prone to variation due to the pressure sensor placement on the experimental circuit while flow is insensitive to the pneumotachograph position. Thus, reporting flow-oscillation amplitude offers a more convenient parameter to allow comparison between studies. Future studies should investigate the impact of this mechanical variable on mucociliary transport.
There are some limitations to this study. We retrieved representative expiratory air-flow waveforms from only 4 subjects and simulated repetitions of these waveforms, which does not account for within-subject variations across an airway clearance session, nor does it represent the wide spectrum of expiratory air-flow waveforms generated by children and adults with different lung disease severities. In addition, we were not able to simulate body temperature and pressure saturated conditions. However, humidity and heat might modify the behavior of OPEP device across consecutive breaths, as is observed in clinical practice. It is not clear if the optimum mechanical parameters elicited by OPEP devices are preserved when they are exposed to body temperature and pressure saturated conditions. Further studies assessing these impacts on the mechanical performance of OPEP devices are warranted. Finally, we focused on mechanical data gathered over the middle 2 parts of the expiratory phase to obtain stable and reproducible signals. This choice may have occulted potential mechanical performance variations between OPEP devices revealed on the whole expiratory phase.33
Conclusions
This simulation study showed some similarities and discrepancies between 6 commonly used OPEP devices. Because the Flutter, Gelomuc, and Pari devices were similar in many aspects, the price may be the incentive to select one of these devices. The variations observed between other OPEP devices must be acknowledged by clinicians to allow for appropriate use. The selection of a particular OPEP device as well as its settings may also depend on whether the clinician prefers to focus on the air-flow oscillation frequency or on oscillation amplitude. Yet the clinical value of these parameters and their relative importance in assisting airway clearance remain to be determined. Further studies assessing the impact of body temperature and pressure saturated expiratory air-flow on OPEP stability are also warranted. The clinical performance of each device is therefore only speculative. At this stage, our findings may guide clinicians to refine the use of OPEP devices to maximize therapeutic efficacy.
Acknowledgments
We thank the MEC Group (Medical Electronic Construction, Brussels, Belgium) for allowing us to use the Hans Rudolph series 1120 flow-volume simulator.
Footnotes
- Correspondence: William Poncin PT PhD, Cliniques universitaires Saint-Luc, Avenue Hippocrate 10, B-1200 Brussels, Belgium. E-mail: william.poncin{at}uclouvain.be.
Dr Poncin is supported in part by French National Cystic Fibrosis Association (Vaincre La Mucoviscidose). The other authos have disclosed no conflicts of interest.
- Copyright © 2020 by Daedalus Enterprises