Abstract
Relevant publications related to medicinal and toxic aerosols are discussed in this review. Treatment of COPD includes a combination of long-acting bronchodilators and long-acting muscarinic antagonists. A combination of aclidinium bromide and formoterol fumarate was approved in the United States. The combination was superior to its components alone, as well as tiotropium and a salmeterol-fluticasone combination. Increased risk of an asthma exacerbation was reported in children exposed to electronic nicotine delivery systems. A smart inhaler capable of recording inspiratory flow was approved in the United States. The use of as-needed budesonide-formoterol was reported to be superior to scheduled budesonide and as-needed terbutaline for the treatment of adults with mild-to-moderate asthma. A survey among teens with asthma and their caregivers revealed a disagreement in the number of inhaled controller medications the teen was taking. Treatment with inhaled hypertonic saline resulted in a decreased lung clearance index in infants and preschool children with cystic fibrosis. Surgical masks were well tolerated and significantly decreased the burden of aerosolized bacteria generated by coughing in adults with cystic fibrosis. Inhaled liposomal amikacin in addition to guideline-based therapy was reported to be superior to guideline-based therapy alone in achieving negative sputum cultures in adult subjects with Mycobacterium avium complex pulmonary disease. During 2019, lung injury associated to e-cigarette or vaping was reported, including 60 casualties.
- aclidinium
- formoterol
- ENDS
- EVALI
- cystic fibrosis
- hypertonic saline
- medication report
- asthma
- COPD
- aerosols
- Pseudomonas aeruginosa
- Staphylococcus aureus
- AMPLIFY
- AFFIRM COPD
- PRACTICAL
- SHIP
- PRESIS
- CONVERT
Introduction
Inhaled therapies are increasingly used to treat pulmonary conditions including COPD, asthma, cystic fibrosis, pulmonary infections with nontuberculous mycobacteria, and others. A PubMed search with the term “aerosol” resulted in 2,375 citations. Relevant reports regarding COPD, asthma, cystic fibrosis, and mycobacterial therapy were published during 2019.
A combination of aclidinium and formoterol was approved by the Food and Drug Administration (FDA) for the treatment of COPD. In addition, a breath-actuated inhaler with smart features was approved by the FDA for the treatment of asthma. A study revealed that teens with asthma and their caregivers have a different recollection of the controller medications the teen received. New studies report improvement in the lung clearance index in infants and preschool children treated with hypertonic saline. New studies have revealed that using surgical masks is well tolerated and decreased the bacterial burden of aerosols generated during a coughing maneuver by adults with cystic fibrosis. An inhaled liposomal formulation of amikacin provided added benefit to guideline-based therapy for the treatment of Mycobacterium avium complex (MAC). In 2019 we witnessed the epidemic of lung injury caused by use of electronic cigarettes.
COPD
Newly Approved Drugs
COPD is a chronic respiratory condition consisting of persistent symptoms and air flow limitation usually caused by significant exposure to noxious gases or particles.1 It is the third leading cause of death in the world. COPD is preventable in most cases and is treatable with new drugs becoming available every year.
In 2019 the FDA approved a combination product of aclidinium bromide and formoterol fumarate for the treatment of COPD. Aclidinium bromide is a long-acting, inhaled muscarinic antagonist that was approved by the FDA in 2012. Formoterol fumarate is a long-acting inhaled bronchodilator that was approved by the FDA in 2001. Their combination is reported to reduce exacerbations more than each component alone according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD).1 Combinations of bronchodilators are recommended in the GOLD guidelines for patients in severe shortness of breath in groups B and D.
Sethi et al2 published the results of a phase 3 study comparing aclidinium/formoterol with the mono-components and with tiotropium in subjects with moderate-to-very-severe symptomatic COPD. The study, titled AMPLIFY, was designed as a randomized, parallel, double-blind, double-dummy, active-controlled, multi-center, multinational phase 3 trial. The study was conducted in 11 countries. Subjects underwent a washout period, followed by a 2-week screening, and then they were randomized to 4 treatment groups for 24 weeks. Subjects were randomized (2:3:2:3) to aclidinium bromide/formoterol fumarate 400/12 μg twice daily (Pressair device), aclidinium bromide 400 μg twice daily, formoterol fumarate 12 μg twice daily, or tiotroprium bromide 18 μg once daily (Handihaler device). Inclusion criteria were age ≥ 40 y, former or current smokers, moderate-to-severe COPD diagnosis according to 2013 GOLD guidelines (postbronchodilator FEV1/FVC < 70% and FEV1 < 80% predicted), and COPD assessment test score ≥ 10 at screening and randomization. Exclusion criteria were respiratory infection or COPD exacerbation within 3 months of screening, or hospitalizations due to COPD exacerbation in the previous 3 months. The primary outcomes were 1-h post-dose FEV1 at week 24 (aclidinium/formoterol vs aclidinium alone), morning pre-dose FEV1 at week 24 (aclidinium/formoterol vs formoterol alone), and morning pre-dose FEV1 at week 24 (aclidinium/formoterol vs tiotropium). A total of 1,594 subjects were randomized, and 85.1% completed the study. Aclidinium/formoterol had greater FEV1 in 1-h post-dose than aclidinium (84 mL), formoterol (88 mL), or tiotropium (92 mL). Aclidinium/formoterol had greater change from baseline in pre-dose FEV1 than formoterol (55 mL), but not with aclidinium (14 mL) or tiotropium (19 mL) monotherapy. The authors concluded that the aclidinium/formoterol combination resulted in greater lung function improvement than either its mono-components or tiotropium.
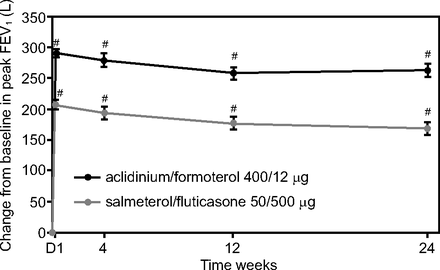
In 2016, Vogelmeier et al3 published the results of a phase 3 study comparing the efficacy and safety of aclidinium/formoterol versus salmeterol/fluticasone in subjects with moderate-to-severe stable COPD. The study was titled AFFIRM COPD, and it was designed as a randomized, double-blind, double-dummy, active-controlled, multi-center trial. The study was conducted in 140 centers in 14 countries. Following a 7–10-d run-in period, subjects were randomized (1:1) to either aclidinium bromide/formoterol fumarate 400/12 μg twice daily (Pressair device) or salmeterol/fluticasone propionate 50/500 μg twice daily (Accuhaler device). Inclusion criteria were age ≥ 40 y, smoking history of ≥ 10 pack-years, and moderate-to-severe COPD diagnosis according to 2013 GOLD guidelines (postbronchodilator FEV1/FVC < 70% and FEV1 < 80% predicted). Exclusion criteria were infection or COPD exacerbation within 3 months of end of run-in period, use of triple therapy (ie, long-acting bronchodilator, long-acting antimuscarinic agent, and inhaled corticosteroid) within 4 weeks before the run-in period, or pulmonary rehabilitation within 3 months. The primary efficacy end-point was peak FEV1 (maximum FEV1 within 3 h of the morning dose) at week 24. A total of 933 subjects were randomized, and 85.9% and 83% completed the study for the aclidinium/formoterol and salmeterol/fluticasone groups, respectively. Subjects in the aclidinium/formoterol group had a peak FEV1 that was 93 mL greater than subjects in the salmeterol/fluticasone group at the end of 24 weeks (Fig. 1). The differences were present since day 1 of therapy, and the differences were greater in subjects who had received inhaled corticosteroids previously or had experienced a COPD exacerbation within the previous 12 months.
Change in aclidinium/formoterol versus salmeterol/fluticasone in COPD from baseline. * P < .0001. From Reference 3, with permission.
Asthma
New Devices
Asthma is a heterogeneous condition characterized by chronic airway inflammation.4 Respiratory symptoms can vary over time, and variable expiratory limitations are present. Access to care, affordability of devices, nonadherence to therapy, and poor inhaler technique constitute 4 significant barriers to effective asthma treatment. A newly developed “smart” inhaler attempts to address the latter 2 barriers. The ProAir Digihaler and the Airduo Digihaler (Teva Pharmaceuticals, North Wales, Pennsylvania) were approved by the FDA on December 2018 and July 2019, respectively (Fig. 2). The devices are breath-actuated, multi-dose, dry-powder inhalers. These devices do not require priming, and they should not be used with either a spacer or a valved holding chamber. The device contains built-in sensors that detect inhaler use and measure inspiratory flow generated during the inhalation maneuver. The data can be sent via Bluetooth to a companion mobile app. This provides the patient the opportunity to share the data with the health care team. The app also offers the possibility of setting reminders to take medications as prescribed. Connection to the app is not required to use the inhaler.
The ProAir Digihaler inhaler records peak inspiratory flow during the inhalation maneuver and it communicates with a smartphone app.
The ProAir Digihaler was approved by the FDA for the treatment of bronchospasm and the prevention of exercise-induced bronchospasm in individuals 4 y of age and older. The inhaler device delivers 117 µg albuterol sulfate per inhalation. The Airduo Digihaler was approved by the FDA for the treatment of asthma in patients 12 y of age or older. It is a combination of fluticasone propionate and salmeterol that is administered as one inhalation twice daily. There are 3 different strengths of fluticasone (55, 113, and 232 µg/inhalation), but the amount of salmeterol is the same in each variation (14 µg/inhalation).
As-Needed Inhaled Corticosteroid/Long-Acting Bronchodilator Therapy
The 2019 asthma clinical practice guidelines developed by the Global Initiative for Asthma recommended the use of low-dose corticosteroid-formoterol as needed for the treatment of mild asthma.4 Hardy et al5 reported the results of a 52-week, open-label, parallel-group, multi-center, randomized, controlled superiority trial comparing budesonide-formoterol reliever therapy versus budesonide as maintenance and terbutaline as a reliever in adults with mild-to-moderate asthma. The study, titled PRACTICAL, was conducted in New Zealand at 15 primary care or hospital-based clinical trial units. The investigators recruited 890 adults with a mean age of 43.3 y; 70% were taking inhaled corticosteroids, 12% had a severe exacerbation in the previous year, and 77% were either partly controlled or uncontrolled according to Global Initiative for Asthma guidelines. Subjects were randomized (1:1) to receive either budesonide 200 μg–formoterol 6 μg (Symbicort Turbuhaler; one inhalation for relief of symptoms as needed) or budesonide 200 μg (Pulmicort Turbuhaler; one inhalation twice daily) plus terbutaline 250 μg (Bricanyl Turbuhaler; 2 inhalations for relief of symptoms as needed). The primary outcome was the number of severe asthma exacerbations per American Thoracic Society/European Respiratory Society criteria per patient per year.6 The authors reported that the rate of severe asthma exacerbations was lower with as-needed budesonide–formoterol than budesonide maintenance plus as-needed terbutaline therapy (absolute rate per patient per year = 0.119 versus 0.172; relative rate = 0.69, 95% CI 0.48–1.00). Time to first severe or moderate-to-severe exacerbation was longer with budesonide–formoterol than with budesonide maintenance plus as-needed terbutaline. Interestingly enough, there were no differences in the asthma control questionnaire between both regimens. These findings provide further support for treatment strategies recommended in the Global Initiative for Asthma guidelines.
Asthma Medication Report in Adolescents and Caregivers
The transition from adolescence to adulthood is a process with several milestones. Adolescents suffering from chronic conditions such as asthma have the additional responsibility of becoming responsible for their daily medical care. A progressive shift of responsibility should occur during adolescence. A survey of parents of children with asthma revealed that by 7, 11, 15, and 19 y of age, children had assumed 20%, 50%, 75%, and 100% of the responsibility for their controller medication, respectively.7 This could potentially create a knowledge gap between the adolescent and the caregiver. Frey et al8 compared the inhaled medications reported by adolescents with persistent asthma with inhaled medications reported by their caregivers. Their data are part of the school-based asthma care for teens program, a clinical trial held in Rochester, New York. The study recruited adolescents 12–16 y old with physician-diagnosed asthma and symptoms consistent with persistent or poorly controlled asthma; subjects attended a partner school. Baseline surveys that asked respondents to name inhaled and rescue medications taken by the teen were completed by adolescents and their caregivers. A total of 210 dyads of adolescents with a mean age of 13.5 y and caregivers with a mean age of 40.6 y were available for analysis. The authors reported a 63% disagreement between teen and caregiver regarding the total number of inhalers used. Furthermore, the authors reported that, in those reporting at least one controller medication (n = 173), there was a 61% disagreement between teen and caregiver regarding the number of controllers used, with 92% of cases not identifying any controlled medication. However, these disagreements did not result in increased symptoms or increased health care utilization.
The findings of Frey et al8 prompt us to rethink the asthma education strategies that we use at different points of care. Providing copies of asthma action plans to both teens and their caregivers might decrease the knowledge gap. These findings also underscore the importance of medication reconciliation during each visit.
Cystic Fibrosis
Hypertonic Saline in Cystic Fibrosis
Cystic fibrosis is an autosomal recessive disease caused by mutations in the cystic fibrosis transmembrane regulator, which plays a prominent role in flow of ions across the cell membrane.9 In the case of the respiratory tract, cystic fibrosis transmembrane regulator malfunction leads to dehydration of the periciliary fluid, with resulting impairment of the ciliary function.10 The use of inhaled hypertonic saline has been recommended for the maintenance treatment of patients with cystic fibrosis > 6 y old, regardless of severity.11 However, data on younger children and infants are scant.
Ratjen et al12 conducted a multi-center, randomized, double-blind, placebo-controlled trial, titled SHIP, comparing 7% versus 0.9% saline inhaled twice daily for 48 weeks in children with cystic fibrosis who were 36–72 months old. The primary outcome was a change in lung clearance index as measured with nitrogen washout from baseline to 48 weeks. A total of 150 preschool children (mean age, 4.5 y) completed the study. The hypertonic group showed a significant treatment difference in the lung clearance index at the end of the study (−0.63, 95% CI −1.10 to −0.15). No differences in time to first exacerbation were found between regimens. Also, the investigators did not consider any of the serious adverse events to be related to the treatment.
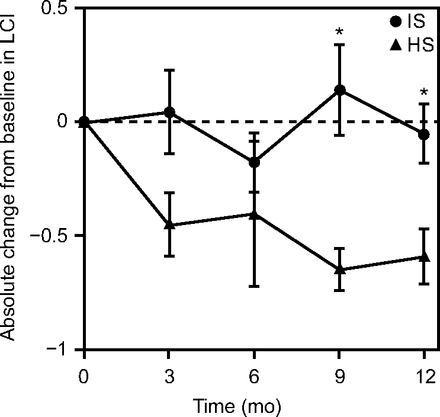
Stahl et al13 reported results of a 12-month, multi-center, double-blind, randomized controlled trial [or RCT], controlled clinical trial comparing safety and efficacy of inhaled 6% hypertonic saline compared to inhaled 0.9% saline solution in infants with cystic fibrosis. The study, titled PRESIS, also tested the feasibility of using the lung clearance index and magnetic resonance imaging as outcome measures for these population. The investigators recruited 42 infants with a mean age of 0.26 y. Upon completion of the trial, the changes in lung clearance index were −0.6 and −0.1 for the 6% and .9% saline groups, respectively (Fig. 3). The imaging scores worsened over time but did not differ between treatment groups. Subjects in the 6% group gained 0.5 kg more weight by the end of the trial than those in the 0.9% group. Height, body mass index, resting breathing frequency, and oximetry were similar between groups at the end of the study. Inhalation was generally well tolerated, and serious adverse events were not rated as related to the study treatment. These results are encouraging because they demonstrated the feasibility of the lung clearance index in evaluating preventive treatments in infants with cystic fibrosis.
Lung clearance index (LCI) in infants treated with 6% or 0.9% inhaled saline solution. Absolute change from baseline in LCI in infants with cystic fibrosis treated with 6% (triangles) and 0.9% (circles) saline solution. Error bars indicate 95% CI. * P < .05. From Reference 13, with permission.
Infectivity of Cough Aerosols in Cystic Fibrosis
Infection-control measures are paramount to prevent and reduce transmission of microorganisms among patients with cystic fibrosis.14 Maintaining distance between 2 patients with cystic fibrosis and disinfection of surfaces after patients with cystic fibrosis are seen as key aspects of the guidelines.14
Wood et al15 reported on survival and traveled distance of microorganisms carried in cough-generated droplets by patients with cystic fibrosis using previously described methodology.16 The authors enrolled adults with cystic fibrosis who grew Gram-negative bacteria and Staphylococcus aureus in their sputum. All subjects with a history of Gram-negative bacteria infection, and 16 of 18 subjects with a history of S. aureus infection, provided an expectorated sample with similar type of bacteria. The mean (95% CI) sputum bacterial concentration (CFU/mL × 106) was 7.0 (95% CI 1.6–31) and 1.3 (95% CI 0.2–7.5) for Gram-negative bacteria and S. aureus, respectively. Cough produced by subjects had 66.5% and 58.2% of particles ≤ 4.7 µm; 83% and 63% of the aerosols of subjects with a history of Gram-negative bacteria and S. aureus infections contained bacteria, respectively. Almost all the cough specimens of the Gram-negative bacteria group, and 50% of the specimens of the S. aureus group were still viable when collected 4 m away from the source and 45 min after having been generated. The sputum bacterial concentration was 12.3 (3–40) and 4 (1–12) for Gram-negative bacteria and S. aureus, respectively.
The same research group also published a report on the effect of face masks and cough etiquette on the propagation of Pseudomonas aeruginosa via cough aerosols in subjects with cystic fibrosis.17 The researchers recruited 25 adults (mean age 31.3 y) with cystic fibrosis who had chronic P. aeruginosa infection. The subjects completed 4 coughing and 2 talking maneuvers: Coughing: (1) uncovered, (2) wearing either a surgical or (3) N95 mask, and (4) hand covering the mouth (cough etiquette); and Talking: uncovered, and wearing a surgical mask. The samples were collected at 2 m to mimic current guideline recommendations.14 The masks were worn for 10 min. Subjects were classified as low or high viable aerosol producers depending on the viable CFUs present during uncovered cough maneuver (< 10 and ≥ 10, respectively). The percentage of particles with size ≤ 4.7 µm that were generated by uncovered cough and cough etiquette maneuvers were 71% and 86%, respectively. Low producers did not recover bacteria during talking with or without a mask, nor while coughing with a surgical mask. However, coughing etiquette and coughing with N95 mask had recovery rates of 40% and 20%, respectively. The recovery rate among high aerosol producers during talking with or without a mask was 8%. The recovery rates during coughing with surgical and N95 masks and with cough etiquette were 14%, 21%, and 79%, respectively. Although wearing either a surgical or a N95 mask provided significant reduction in aerosol transmission of P. aeruginosa, the effect of using cough etiquette was only partial.
The study in which masks were worn for 10 min17 was followed by another study testing the effect of time wearing a mask on its ability to reduce the growth of bacteria.18 The authors compared CFUs produced by an uncovered cough with those produced while wearing a surgical mask for 10, 20, and 40 min, and while wearing an N95 mask for 20 min. The authors reported that the CFU count was similar among different lengths of mask use. The investigators also compared comfort of use (ie, poor, sufficient, good) and the mask weight in subjects with cystic fibrosis and in a control group. They reported that subjects with cystic fibrosis reported less comfort with a surgical mask than healthy controls, with the latter having more responses in the good category as opposed to the subjects with cystic fibrosis, who had more responses in the sufficient category. The N95 mask was poorly tolerated by subjects with cystic fibrosis after 20 min of use. The surgical mask weight increased with use time, but this reached statistical significance only when comparing 10 min of use to 40 min of use. The findings from this research group17,18 support guideline recommendations to use masks to decrease transmission of microorganisms among patients with cystic fibrosis.14
Liposomal Amikacin for MAC Lung Disease
Nontuberculous mycobacterial pulmonary disease af-fects both heathy individuals and those with underlying lung disease.19 Its prevalence is increasing and is estimated to be 23–37 cases per 100,000 individuals. An article on the state of the art of nontuberculous mycobacterial pulmonary disease was recently published.19 MAC bacteria comprise the most common etiology of nontuberculous mycobacteria pulmonary infections.19,20 Current guidelines recommend a 3-antibiotic regimen with the goal of achieving negative cultures by 12 months of therapy.20 There are no treatment alternatives for those who fail guideline-based therapy. Griffith et al21 reported the results of an open-label, non–placebo-controlled, randomized (2:1), clinical trial, titled CONVERT, of amikacin liposome inhalation suspension in addition to guideline-based therapy versus guideline-based therapy alone in adults with amikacin-sensitive MAC lung disease who were still positive after 6 months of guideline-based therapy. The treatment consisted of amikacin 70 mg/mL encapsulated in dipalmitoyl phosphatidylcholine and cholesterol, with 590 mg/8.4 mL amikacin delivered once daily with an investigational eFlow nebulizer (Lamira, PARI Pharma GmbH). The outcome measure was the proportion of subjects who were culture-negative at the end of 6 months. A total of 336 subjects were recruited with mean age of 64.7 y, and female predominance (69.3%), with most subjects having bronchiectasis without COPD as their main diagnosis (62.5%). Subjects receiving inhaled amikacin were more likely to achieve negative cultures (hazard ratio 3.90, 95% CI 2.00–7.60) (Fig. 4). Serious adverse events were comparable in both groups. The drug was approved by the FDA in September 2018 and included a black box warning of increased respiratory adverse reactions (eg, hypersensitivity pneumonitis, hemoptysis, bronchospasm, and exacerbation of underlying pulmonary disease).
Liposomal amikacin for the treatment of mycobacterium avium complex lung disease. Proportion of subjects with culture conversion over time: intention-to-treat-population. ALIS = amikacin liposome inhalation; GBT = guideline-based therapy. From Reference 21, with permission.
Electronic Nicotine Delivery Systems
E-Cigarette or Vaping Associated Lung Injury
Electronic nicotine delivery systems (ENDS) generate an aerosol by heating a liquid.22 They were first introduced in the United States in 2007, and their use rapidly increased in part due to lack of early regulation by the FDA. The liquid contains many ingredients including flavoring, propylene glycol, vegetable glycerin, and other ingredients. These devices are also used to deliver tetrahydrocannabinol (THC), the psychotropic component of marijuana. Although the prevalence of use in high school students was low in 2011 (1.5%), it significantly increased by 2015 (16%).23 There was a transient decrease in prevalence to the low teens during 2016 and 2017, but use nearly doubled in 2018.23 Its prevalence among high school and middle school students was reported to be 27.5% and 10.5%, respectively, in 2019.24
On August 1, 2019, the first cluster of lung injuries later associated with the use of ENDS was reported to the Centers for Disease Control and Prevention (CDC). The condition was termed e-cigarette, or vaping, product use associated lung injury. The CDC developed criteria to identify confirmed and probable cases.25 The criteria for confirmed cases included using an e-cigarette (vaping) or dabbing 90 d prior to symptom onset, presence of pulmonary infiltrate such as opacities on plain film chest radiograph or ground-glass opacities on chest computed tomography, absence of pulmonary infection on initial work-up, and no evidence in medical record of alternative plausible diagnoses (eg, cardiac, rheumatologic, or neoplastic process). Probable cases had similar criteria except that an infection was identified via culture or other techniques, but the clinical team believed that this was not the sole cause of the lung injury. Butt et al26 reviewed the lung biopsies of 17 subjects with confirmed and possible e-cigarette, or vaping, product-use associated lung injury. They did not observe specific histologic findings, but they found foamy macrophages (also in bronchoalveolar lavage) and pneumocyte vacuolization in all cases. Neutrophils were often prominent, but eosinophils were rare.
By November 5, 2019, 2,051 cases including 39 deaths in 24 states had been reported. Most patients reported a history of using products containing THC. Most patients were males under the age of 35 y. Almost half of these cases were in people < 21 y old. As of January 14, 2020, the CDC reported 2,668 cases of e-cigarette, or vaping, product use associated lung injury with a median age of 24 y (76% of patients are ≤ 35 y old) and a male predominance (66%). Most subjects (82%) reported using a THC-containing e-cigarette or vaping product.27,28 One third of the subjects reported exclusive use of THC-containing products, whereas 14% of patients reported exclusive use of nicotine-containing products. Cases were reported in all 50 states, the District of Columbia, the United States Virgin Islands, and Puerto Rico. Vitamin E acetate was reported to be present in the bronchoalveolar lavage of patients suffering from e-cigarette, or vaping, product use associated lung injury.29 The number of reported deaths increased to 60 by January 21, 2020, but more deaths are under investigation.
Secondhand Exposure
Secondhand exposure to vapors generated by ENDS has been considered harmless. However, a recent report by Bayly et al30 depicted a different reality. The authors analyzed the 2016 Florida youth tobacco survey, which included 11,830 subjects with self-reported diagnosis of asthma aged 11–17 y. The survey assigned exposure status if the respondent had been either in the same room or had ridden in a car with somebody smoking either cigarettes or electronic vapor products during the previous 30 d. The respondents reported secondhand exposure rates to tobacco and ENDS of 45.4% and 32.8%, respectively. The authors reported an increased odds of having an asthma exacerbation in the past 12 months, with adjusted odds ratios of 1.19 (95% CI 1.05–1.35) and 1.27 (95% CI 1.11–1.47) for tobacco and ENDS exposure, respectively. The findings of this study should help change our practice so that we discuss the consequences of ENDS exposure with all of our patients. This is more relevant for those caring for pediatric patients.
Summary
The combination of long-acting bronchodilators and long-acting antimuscarinic agents was more effective for the treatment of COPD than either component alone or a long-acting bronchodilator/corticosteroid combination. The role of education in the management of asthma is paramount. Newer technologies could potentially help improve inhalation technique and treatment adherence. The use of budesonide/formoterol as needed was more effective than the use of maintenance budesonide and as-needed terbutaline in preventing moderate-to-severe and severe asthma exacerbations. The use of hypertonic saline in infants and preschool children with cystic fibrosis resulted in a decrease of the lung clearance index. The use of masks reduced the transmission of microorganisms in subjects with cystic fibrosis. Treatment with liposomal inhaled amikacin in addition to guideline-based therapy was superior to guideline-based therapy alone in obtaining negative cultures from the sputum of subjects with MAC lung disease. The use of ENDS has significantly increased in adolescents. The use of these devices was responsible for morbidity and mortality due to lung disease (ie, e-cigarette, or vaping, product-use associated lung injury). Secondhand exposure to ENDS resulted in an increased risk of having an asthma exacerbation. These publications highlight the important role of respiratory therapists in preventing and treating lung disease in children and adults.
Footnotes
- Correspondence: Ariel Berlinski MD FAARC, Department of Pediatrics, 1 Children's Way, Little Rock, AR 72202. E-mail: berlinskiariel{at}uams.edu
Dr Berlinksi presented a version of this manuscript at the American Association of Respiratory Care Congress, held November 9–12, 2019, in New Orleans, Louisiana.
Dr. Berlinski has disclosed relationships with AbbVie, Allergan, Anthera, DCI, Cempra, Cystic Fibrosis Foundation, National Institute of Health, Novartis, Therapeutic Development Network, Trudell Medical International, Vertex and Vivus, as well as the International Pharmaceutical Aerosol Consortium on Regulation and Science (IPAC-RS).
- Copyright © 2020 by Daedalus Enterprises