Abstract
Respiratory compromise is a common and potentially dangerous complication of patients admitted to general care units of hospitals. There are several distinct and disparate pathophysiologic trajectories of respiratory deterioration that hospitalized patients may suffer. Obstructive sleep apnea and preexisting cardiopulmonary disease increase the risk of respiratory failure after major surgery. Patients in general care units of hospitals currently receive only intermittent monitoring of vital signs. Early warning systems that utilize analysis of intermittently collected vital signs may result in earlier recognition of clinical deterioration. Continuous monitoring of oximetry and capnography may allow the detection of pathophysiologic abnormalities earlier in patients in general care units, but the evidence for improved clinical outcomes remains weak. Increased monitoring may lead to increased monitor alarms that can have negative effects on patient care.
- monitoring
- physiologic
- vital signs
- clinical deterioration
- respiratory insufficiency
- conscious sedation
- hospital rapid-response team
- biomedical informatics
Introduction
Most patients in ICUs in acute care hospitals are connected to multiple sensors providing nearly continuous monitoring of pulse, blood pressure, breathing frequency, and oxygen saturation. Robust health care staffing and monitor alarms permit early intervention in response to clinical deterioration in patients in the ICU. In contrast, patients in general care units experience only intermittent collection of vital signs and perhaps pulse oximetry. If one estimates that collection of intermittent vital signs by staff requires 5 min, even vital signs gathered every 4 h corresponds to monitoring only 2% of the time. Staffing is significantly lower in general care units, and there are various thresholds of abnormal vital signs that would trigger a response from the hospital’s medical emergency team.
Patients cared for in the general care units of hospitals are at significant risk for respiratory compromise. Respiratory compromise is the deterioration in respiratory function with a high likelihood for progression to respiratory failure or death but for which timely specific interventions (eg, enhanced monitoring or therapies) might prevent or mitigate decompensation.1 The annual incidence of respiratory compromise in general care units for U.S. hospitals is estimated to be 44,500–64,000.2,3 It is estimated that 41% of all in-hospital cardiac arrests occur outside of the ICU.4
The most common cause of postoperative mortality is respiratory complications. The Agency for Healthcare Research and Quality rated postoperative respiratory failure as the fourth most common patient safety event in U.S. hospitals in its 2015 report.5 A prospective multi-center observational study in patients undergoing noncardiothoracic surgery with severe systemic disease (American Society of Anesthesiologists physical status ≥ 3) documented that 3.8% of these subjects required postoperative noninvasive ventilation and 1.7% required re-intubation and postoperative invasive ventilation.6 Subjects requiring re-intubation and invasive ventilation after surgery demonstrated a marked increase in 7-d mortality (relative risk 28.0, interquartile range 7.5–104.6, P < .001).
Medical in-patients have an incidence of 0.91% of developing respiratory insufficiency, arrest, or failure that was not present on admission, leading to a hospital mortality of 34.6%. Respiratory failure not present on admission results in prolonged ICU stay and hospital stay, as well as significant increases in total hospital costs.7 Medical in-patients diagnosed with acute respiratory failure > 24 h after hospital admission have a higher in-hospital mortality than patients diagnosed with acute respiratory failure that was present on admission (32.7% vs 27.8%, P < .001).8
Respiratory Compromise
A recent workshop described several distinct pathophysiologic subsets of respiratory compromise: impaired control of breathing, impaired airway protection, parenchymal lung disease, increased airway resistance, hydrostatic pulmonary edema, and right-ventricular failure.1 The report emphasized that the clinical presentation may differ based on the underlying disease. Respiratory compromise can occur in patients with impaired control of ventilation due to central nervous system injury or respiratory depression from drugs such as opioids, leading to progressive hypercarbia. Severe sleep apnea can inhibit central control of breathing or produce upper-airway obstruction, leading to progressive hypercarbia and, rarely, sudden cardiac death. Progressive parenchymal lung disease (such as pneumonia, atelectasis, or ARDS) leads to ventilation-perfusion mismatch and decreased lung compliance. Patients with parenchymal lung disease will exhibit tachypnea, increased work of breathing, and hypoxemia. Patients hospitalized with COPD or asthma are at risk for respiratory compromise due to increased airway resistance, which causes air-trapping and increased work of breathing. Initially patients with increased airway resistance are tachypneic, but bradypnea and respiratory acidosis may manifest as respiratory compromise advances. Patients with hydrostatic pulmonary edema present with tachypnea, increased work of breathing, and hypoxemia. Respiratory compromise from right-ventricular failure due to acute pulmonary hypertension or pulmonary arterial hypertension will present with tachypnea and hypoxemia, but diagnostic tests specific to right-ventricular function (eg, echocardiogram or elevated cardiac biomarkers) are required for diagnosis and prognosis. Different pathophysiologic states have disparate clinical manifestations that may require monitoring of different physiologic parameters.
Trajectories of Unexpected Hospital Death
To understand monitoring in the general care unit, it is critical to understand the pathways that patients take from progressive respiratory compromise to respiratory failure to death. Lynn and Curry9 described 3 separate and distinct trajectories that may culminate in unexpected hospital death. The most common pathway occurs in patients with sepsis, congestive heart failure, or pulmonary embolism. The earliest physiologic response to microcirculatory failure in these processes is hyperventilation with an increase in minute ventilation due to an increase in both breathing frequency and tidal volume with preservation of oxygen saturation (Fig. 1). During this hyperventilation phase, focusing solely on an oxygen saturation > 90% often leads to a false sense of security and delayed recognition of clinical deterioration. As respiratory compromise evolves in this pathway, a metabolic acidosis often ensues, leading to further tachypnea. This pathophysiology leads to a divergence of increased breathing frequency and decreasing oxygen saturation. If supplemental oxygen is delivered, oxygen saturation can remain normal until shortly before death in this potentially lethal pathway.
Hyperventilation compensated respiratory distress such as sepsis, pulmonary embolism (PE), or congestive heart failure (CHF).  = minute ventilation. From Reference 9.
= minute ventilation. From Reference 9.
Opioids can lead to progressive hypercarbia due to decrease minute volume, primarily due to a reduced breathing frequency. A decrease in pulse oximetry may be noted as oxygen is displaced at the alveolar level by an increasing concentration of carbon dioxide. However, supplemental oxygen is often administered in hospitalized patients, which permits marked elevation of carbon dioxide before a low oxygen threshold is breached. Supplemental oxygen blunts the potential early warning sign of reduced oxygen saturation in progressive hypercarbia due to opioids (Fig. 2).
Progressive hypoventilation (CO2 narcosis).  = minute ventilation. From Reference 9.
= minute ventilation. From Reference 9.
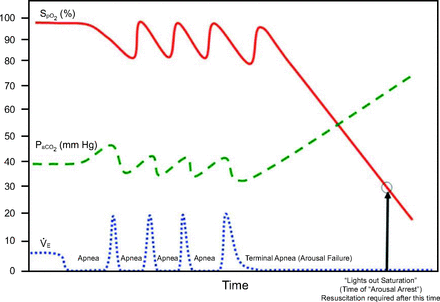
The third and potentially more lethal pathway can occur in patients with obstructive sleep apnea (Fig. 3). Obstructive sleep apnea can be viewed as a series of repetitive collapses of the upper airway due to decreased muscular tone that are followed by central nervous system arousal. There is repetitive decrease in air flow and oxygen saturation during sleep in patients with obstructive sleep apnea. Opioids and sedatives can increase the threshold for central nervous system arousal, preventing airway opening and leading to a rapid increase in carbon dioxide and a rapid decrease in oxygen saturation.10 This pathophysiology has been implicated in sudden death in patients with obstructive sleep apnea.11
Sentinel airflow/hypoxemia followed by arousal arrest.  = minute ventilation. From Reference 9.
= minute ventilation. From Reference 9.
Risk Factors for Respiratory Compromise
Understanding risk factors for respiratory compromise may allow the application of monitoring techniques for patients at increased risk. There are several validated preoperative risk scores to predict postoperative respiratory failure. For example, ARISCAT (Assess Respiratory Risk in Surgical Patients in Catalonia) uses age, preoperative O2 saturation, surgical incision (upper abdomen or thoracic), duration of surgery, and other clinical risks (eg, anemia, recent respiratory infection, or emergency surgery).12 There are no prospective studies on the utilization of preoperative risk scores to direct the application of enhanced monitoring.
A retrospective analysis demonstrated an association between opioid analgesic and sedative medications and in-hospital cardiac and cardiopulmonary arrest in both medical and surgical hospital in-patients.13 Obstructive sleep apnea increases the risk of postoperative respiratory failure (odds ratio 2.42, 95% CI 1.53–3.84, P < .001) and ICU transfer (odds ratio 2.46, 95% CI 1.29–4.68, P = .006).14 Preexisting cardiopulmonary disease increases the likelihood of postoperative respiratory failure. For example, patients with COPD have an increased risk for postoperative respiratory failure (5.5% vs 1.2%, P < .001).15
A recent prospective analysis identified several predictors (P < .05) of postoperative respiratory depression in a univariate analysis: age, male sex, major organ failure, chronic heart failure or cardiac disease, coronary artery disease, COPD or pulmonary disease, pneumonia, type II diabetes mellitus, hypertension, kidney failure, and opioid naivety. The authors proposed a risk-prediction score utilizing age, male sex, sleep disorder, hypertension, and opioid naivety. However, the postoperative respiratory depression prediction score performance was only fair, predicting 76% of subjects with confirmed respiratory depression.16
Early Warning Systems
Clinical deterioration is defined as “movement from one clinical state to a worse clinical state which increases the individual risk of morbidity or death.”17 Using activation of a medical emergency team as a surrogate for deterioration, there is a 3–9% incidence of clinical deterioration in general care units.17 In-hospital cardiac arrest on general care units is frequently preceded by abnormal vital signs, with one study documenting 59% of subjects having abnormal vital signs 1–4 h before arrest.18 In a retrospective study using a validated early warning score, there was a 3% increase in mortality for each hour of delay in transfer to ICU.19
Rapid-response systems have multiplied in U.S. hospitals over the past 2 decades. In 2004, the Institute for Healthcare Improvement began its “100,000 Lives Campaign,” which called for all U.S. hospitals to deploy rapid-response teams.20 Rapid-response teams have been promulgated in hospitals over this period with debate regarding their effectiveness on clinical outcomes.21 Rapid-response teams are part of the efferent or the response limb of a rapid-response system, and monitoring and early warning scores represent the afferent limb (Fig. 4).22
Physiologic deterioration and opportunities for intervention. From Reference 21.
The afferent limb of the rapid-response system includes monitoring for an abnormality in physiologic variable(s), recognition of the abnormality, and triggering a response when a certain threshold is breached. For example, a breathing frequency > 27 breaths/min in the prior 72 h is most predictive of cardiac arrest in medical patients in general care units.23 Machine learning on a large database (n = 270,000 subjects) demonstrated that breathing frequency (low or high) was the variable that was most predictive of the combined outcome of cardiac arrest, ICU transfer, or death.24
The afferent or detection limb on the in-patient hospital units is evolving rapidly with recent technological advances. Nearly universal adoption of hospital electronic health records in U.S. hospitals allows the collection and analysis of vast amounts of data. There has been significant progress in physiologic sensors, which allows improved collection of data, sometimes without physical contact. Improvements in mobile technologies permit wireless assimilation of vital signs as well as notification of health care providers. Machine learning of large amounts of data permits improved prediction of clinical deterioration compared to standard statistical regression analyses.25
Analysis of intermittent vital signs by using early warning scores may permit earlier recognition of deterioration. A recent review concluded that intermittent vital sign monitoring coupled with early warning scores predicted cardiac arrest and death within 48 h, but the impact on health outcomes and resource utilization remains uncertain.26 Studies of intermittent vital sign monitoring involving different frequencies of vital sign collection, variable early warning triggers, and disparate emergency responses failed to demonstrate improvement in the prevention of cardiac arrest, reduction in the length of hospital stay, or prevention of other neurological or cardiovascular adverse events. 27 A before-and-after study utilizing an automated notification system exhibited a significant decrease in cardiac arrests and overall mortality.28 Automated intermittent vital sign collection coupled with increased computational power permits vital sign trend analyses that may increase the predictive value for clinical deterioration.29
Continuous Monitoring
An alternative monitoring approach to intermittent vital sign collection is the utilization of continuously collected physiologic parameters. When patients have their vital signs checked every 4 h for 5 min at a time, overall they are monitored only 2% of the time. A recent large database review documented frequent errors, omissions, and outliers in vital sign measurements that were recorded hourly in ICUs. The authors found that 15–38% of vital sign days contained at least one statistical outlier of a vital sign.30 In an analysis of closed claims for postoperative opioid respiratory depression, 78% of the events occurred within 2 h of the last collection of vital signs.31 The most widely studied continuous monitoring techniques are pulse oximetry and capnography. A study of intermittent manual charting compared with automated continuous monitoring of pulse oximetry indicated that documented oxygen saturation was frequently incorrect; the manually charted pulse oximetry measurements were higher on average by 6.5% (95% CI 4.0–9.0).32
Oximetry
A large, randomized controlled study (n = 20,802) in the operating room and postanesthesia care unit revealed that pulse oximetry detects hypoxemia (ie, oxygen saturation < 90%) significantly better than without oximetry (7.9% vs 0.4%, P < .001). Myocardial ischemia was more common in the control group than in the oximetry group; however, pulse oximetry did not decrease the rate of postoperative complications or mortality.33 A recent study indicated that postoperative hypoxemia is common and persistent (ie, 37% of subjects had at least one  measurement < 90% for ≥ 1 h) and goes unrecognized (90% of prolonged hypoxemic episodes were not recorded by bedside nurses).34 A before-and-after study of continuous pulse oximetry in a postoperative orthopedic ward documented a decrease in rescue events from a mean (SD) of 3.4 (1.89–4.85) to 1.2 (0.53–1.88)/1,000 patient discharges (P = .01).35 ICU transfers decreased from a mean (SD) of 5.6 (3.7–7.4) to 2.9 (1.4–4.3)/1,000 patient days (P = .02) with continuous oximetry monitoring. Two comparison hospital units had no change in rescue events or ICU transfers.35 The only randomized controlled trial of continuous pulse oximetry in a general care unit failed to demonstrate an overall decrease in ICU transfer, mortality, or total estimated costs of hospitalization.36 A Cochrane meta-analysis concluded that pulse oximetry reduces the incidence of hypoxemia but does not improve overall patient outcomes and does not reduce morbidity or mortality.37 As noted in Figure 2, pulse oximetry in patients receiving supplemental oxygen may remain in the normal range despite significant hypoventilation. Because of this shortcoming of pulse oximetry, attention has been placed on measures of ventilation, such as capnography.
measurement < 90% for ≥ 1 h) and goes unrecognized (90% of prolonged hypoxemic episodes were not recorded by bedside nurses).34 A before-and-after study of continuous pulse oximetry in a postoperative orthopedic ward documented a decrease in rescue events from a mean (SD) of 3.4 (1.89–4.85) to 1.2 (0.53–1.88)/1,000 patient discharges (P = .01).35 ICU transfers decreased from a mean (SD) of 5.6 (3.7–7.4) to 2.9 (1.4–4.3)/1,000 patient days (P = .02) with continuous oximetry monitoring. Two comparison hospital units had no change in rescue events or ICU transfers.35 The only randomized controlled trial of continuous pulse oximetry in a general care unit failed to demonstrate an overall decrease in ICU transfer, mortality, or total estimated costs of hospitalization.36 A Cochrane meta-analysis concluded that pulse oximetry reduces the incidence of hypoxemia but does not improve overall patient outcomes and does not reduce morbidity or mortality.37 As noted in Figure 2, pulse oximetry in patients receiving supplemental oxygen may remain in the normal range despite significant hypoventilation. Because of this shortcoming of pulse oximetry, attention has been placed on measures of ventilation, such as capnography.
Capnography and Other Measures of Ventilation
Measurement of exhaled carbon dioxide (ie, capnography) can be performed in spontaneously breathing patients utilizing the sidestream technique.38 Capnography allows the measurement of air flow and breathing frequency as well as end-tidal carbon dioxide concentration. A recent meta-analysis using pooled data from 3 studies indicated that continuous capnography identified postoperative respiratory depression (ie, oxygen desaturation, bradypnea, or hypercarbia) in 11.5% of postoperative subjects versus 2.8% (P < .001) of subjects monitored with continuous oximetry.39 No studies included in the meta-analysis examined the impact of continuous capnography on reducing rescue team activation, ICU transfers, or mortality.
Spontaneous ventilation is an important physiologic parameter that is complementary to pulse oximetry. There are several alternative technologies to capnography that measure the adequacy of spontaneous ventilation. Respiratory-induced variation in the photoplethysmography of pulse oximetry permits measurement of breathing frequency.40 A bioacoustic monitor of air flow can reliably monitor breathing frequency.41 Chest wall movement can be measured with plethysmography technology (ie, elastomeric, impedance, or inductive).42 A wearable patch utilizes an accelerometer to measure breathing frequency.43 A piezoelectric element under a patient’s mattress can noninvasively measure breathing frequency as well as heart rate.44
Procedural Sedation and Analgesia
Procedural sedation and analgesia refer to the relief of pain and anxiety to allow patients to tolerate uncomfortable diagnostic or therapeutic procedures. Sedation is also utilized to facilitate procedures in which immobility may be necessary (eg, pediatric radiographic studies). With increased use of gastroenterology and pulmonary endoscopy, interventional radiology, and cardiac procedures, the fraction of anesthesia delivered outside of the operating room has increased significantly from 28.3% in 2010 to 35.9% in 2014 (P < .001).45 An analysis of closed malpractice claims documented that respiratory mechanisms were more common outside the operating room (44% vs 20%, P < .001), with inadequate oxygenation or ventilation being the most common event (ie, 21% outside the operating room vs 3% in operating room, P < .001). The proportion of claims for death was increased in remote location claims (54% vs 29% for operating room claims, P < .001). Remote location claims were more often adjudicated as being preventable with better monitoring (32% vs 8% for operating room claims, P < .001).46
Sedation and analgesia comprise a continuum, with spontaneous ventilation deemed adequate with moderate sedation but often inadequate in patients receiving deep sedation (Table 1).47 In a study of intended moderate sedation (with intravenous meperidine and midazolam) for gastroenterology endoscopy, 68% of subjects actually attained deep sedation.48
Levels of Sedation
Capnography has been reported to provide earlier indication of apnea than continuous pulse oximetry during gastrointestinal endoscopy with procedural sedation.49,50 A meta-analysis in 201151 concluded that episodes of respiratory depression were 17.6 times more likely to be detected with capnography than with standard monitoring. A recent meta-analysis that included 6 studies concluded that capnography reduced hypoxemic events (defined as < 90% saturation; relative risk 0.71 (95% CI 0.56–0.91, P = .02), but there was no conclusive evidence for effect on clinical outcomes, such as assisted ventilation.52 Another meta-analysis of 13 studies reached the conclusion that capnography reduces episodes of severe hypoxemia (defined as ≤ 85% saturation; odds ratio = 0.55, 95% CI 0.38–0.78) and the need for assisted ventilation (odds ratio 0.47, 95% CI 0.23–0.95).53 Despite the lack of compelling evidence of differences in clinical outcomes with the use of capnography for procedural sedation, multiple national organizations have authored guidelines recommending its use.54 The American Society of Anesthesiologists’ guideline recommends continuous monitoring of ventilatory function by observation and with capnography unless precluded or invalidated by the nature of the patient, procedure, or equipment.55
Alarm Fatigue and Negative Impact of Monitoring
Despite their potential benefits, alarms can also have negative effects. Alarm fatigue is defined as failure to recognize and respond to true alarms that require intervention as a result of high occurrence of alarms. The Joint Commission issued a Sentinel Event Alert in 2013 implicating alarm fatigue in 98 adverse events, including 80 deaths.56 ECRI Institute has rated alarm problems among the top 10 health technology hazards, calling hospital alarm issues “the number one medical hazard of 2014.”57 Monitoring patients in the general care unit is more challenging than in the ICU because of increased patient movement and a lack of patient acceptance of attached sensors. Postsurgery floors and medical floors are different than an operating room, where the oximetry threshold breach of 90% has been utilized for decades. Patients in the operating room are anesthetized and have an artificial airway in place, such that a drop in oxygen saturation < 90% is critical. Many postsurgical patients suffer self-correcting innocent sleep apnea with brief, repetitive desaturations. Postsurgical patients spend 6% of their sleep time with oxygen saturation < 90%.35 There is an inherent tradeoff of alarm sensitivity and false alarms. Taenzer et al35 utilized an oxygen saturation of 80% as the lower threshold alarm in their clinical trial of continuous monitoring. The addition of appropriate notification delays can also decrease false alarms. There is a movement to redesign hospital systems to a biologically valid, clinically relevant, and patient-centered model that serves to improve patient safety.58
Continuous patient monitoring in general care units may have a negative impact on patient outcomes. Continuous pulse oximetry may identify mild hypoxemia in otherwise stable infants with bronchiolitis. Randomized clinical trials have indicated that mild hypoxemia leads to an increased rate of hospitalization and prolongation of hospital stay without an improvement in clinical outcomes.59,60 The recent American Academy of Pediatrics clinical practice guideline on the management of bronchiolitis advised against the routine use of continuous pulse oximetry.61
Summary
Respiratory compromise is common and is not always clinically appreciated in general care units within hospitals. Patients move from respiratory compromise to respiratory failure along different trajectories on the basis of their underlying pathophysiologic derangements. The collection of intermittent vital signs coupled with a validated early warning system may allow earlier detection of clinical deterioration. The evidence for routine continuous pulse oximetry and capnography in general care units is weak. Advanced sensor technology and improved integration of clinical data may improve early detection of deterioration and clinical outcomes. The area of monitoring in general care units represents an unmet need that is ripe for innovation and research.
Discussion
*Myers:
Jim, nice presentation and it’s an area of concern because it’s outside of the ICU that probably doesn’t have a lot of monitoring. Interestingly, at the beginning of your presentation you showed data that a lot of these patients with respiratory compromise, respiratory failure, and so forth are not traditional patients with respiratory disease and therefore are not being seen by RTs on a routine or regular basis. Based on a presentation yesterday regarding the inability to document or appropriately document breathing frequency, which may be one of the best indicators, that becomes very problematic. What are your thoughts about those patients?
Lamberti:
Tim, that’s a good thought. When we designed the retrospective Medicare claims review,1 we thought it would be COPD patients who developed respiratory failure in the hospital. It wasn’t. These are patients with renal failure, cardiac disease, and other problems leading to respiratory failure. Acute renal failure was the biggest predictor of respiratory compromise in the hospital. I think that we have blinders that we need to remove. I think RTs should be at the forefront with clinical assessment of many of these patients. There should be a trigger that says, ‘ok this patient needs a consultation with an RT based on these findings.’
Blanch:
In line with what you said, how can this be improved by adding measurements like lactate or rapid response teams?
Lamberti:
I think machine learning will help us with these types of decisions. Lactate was analyzed in the machine learning study and it was pretty far down in predictive value for ICU transfer or death.2 I think what we need to do is have specific analytics for specific patients; lactate will help in presumed sepsis. Serum lactate will not help in some other disease states. We need to figure out, by using big datasets, what information will help us the most. We’re not there yet. It’s unfulfilling because at this time, I can’t give you an answer of who should get what monitoring. Just as you presented, Lluís [Blanch] – we have to move further into integration of all this data that we have in hospitals. We have so much information that is not in our electronic health records.
Blanch:
I agree. We have a lot of information which is not used. We also need a continuum of this information and trends interpretation when the patient is moving within the hospital.
Lamberti:
There’s a nice paper that looked at vital sign trends.3 Trends are even more predictive of cardiac arrest than an individual set of vital signs. But as a individual caregiver at the bedside, it’s difficult to decipher a trend. We need to utilize machine learning. We need to integrate a lot of different data and say, “based on this trend, it is likely that clinical deterioration will occur.” Blanch, I couldn’t agree more.
Blanch:
There’s another paper coming out regarding similar issues in sepsis,4 so there is no reason to think that this can’t also happen in monitoring.
Lamberti:
Absolutely.
MacIntyre:
You bounced back and forth, as all of us do, between respiratory compromise as a sort of single entity as opposed to an entity with multiple causes and trajectories. As you showed very nicely, Jim [Lamberti], it is clearly not a single entity. A pulmonary embolism is a whole lot different than a stroke, and yet you end up with emergent intubation with both of them. But the trajectory, the predictions, the patterns, the monitoring strategies for those two events are totally different. It makes this whole field to me a very challenging one.
Lamberti:
I think we need to take a step back. We tend to simplify complex physiology. There was a recent study in sepsis about clustering of 4 different phenotypes.4 That’s what we need to do in respiratory compromise leading to respiratory failure. I think analyzing data (like Dr MacIntyre looking at unexpected extubations at Duke) will allow us to define different trajectories of respiratory compromise. We have to work with our computer colleagues. One of the problems is to simplify it for clinicians. We say a breathing frequency >27 breaths/min is bad; but breathing frequency <6 breaths/min is bad too. It depends on the clinical scenario. Neil, I think that’s a great point.
Scott:
It seems, between my presentation, this one, and some of the other conversations, that in some patients we have an over-reliance on pulse oximetry and under-reliance on monitoring of breathing frequency. Moving forward, we need to figure out how to draw that data out.
Lamberti:
I couldn’t agree more. Pulse oximetry, particularly with supplemental oxygen, can stay normal until late in the course of respiratory failure. In the University of Chicago machine learning paper, pulse oximetry was only 1/3 as important as breathing frequency to predict cardiac arrest or ICU transfer.2 We need to get that message out. Caregivers need to hear that you can deteriorate and potentially die from respiratory failure with a normal SpO2.
Scott:
I think as educators and thought leaders, we need to focus less on what pulse oximetry does and focus more on what it doesn’t do.
Lamberti:
Yes. Those diagrams of trajectories of unexpected hospital death are not well publicized.5 The trajectories are hypothetical and stylized, but emphasize the importance of viewing respiratory rate, mental status, pulse oximetry and end-tidal PCO2.
Rackley:
How do you balance the timing of these measurements and everything else that you’re monitoring with staffing?
Lamberti:
Great point. We need to have some kind of automated systems. There are studies that show you don’t want to wake patients up at night, it affects sleep patterns. There are staffing limitations on general care units. You need to balance obtaining meaningful data with staffing limitations versus bothering the patient. I think that the answer in the future will be wearables.
Rackley:
That was my next question. Do you now have almost all of your vitals and monitoring automated so that the nurses and RTs are not going in as much for routine monitoring? They can then focus more of their energy on the few patients with concerning trajectories as opposed to spending all of their time collecting these data for all of the patients.
Lamberti:
My concern is we can’t lose sight that there is still a patient. Visual connection with our patients is important. But, we need to focus on who needs us and who doesn’t. With the current model, everybody gets the same battery of vital signs. In the general care unit, we might increase the frequency of vital signs to q 2 hours if we’re concerned; but our monitoring is still intermittent.
†Hess:
I was struck that you presented the evidence for capnography and procedural sedation and it appeared that the evidence wasn’t very strong. Then the next slide came up, and it was, ‘the ASA says you should use it continuously/continually.’ I don’t get it. Why that disconnect? Did I miss something?
Lamberti:
Dean, you didn’t miss anything. I set the presentation up that way on purpose. I think it made the point that the RCT data is weak. If you compare us to the aviation safety literature at a certain point there has to be some belief that physiology and common-sense matters. The RCT isn’t always the right modality, I agree with you.
MacIntyre:
We recommend parachutes without a randomized trial!
Lamberti:
There you go.
†Hess:
You can question whether you need a randomized trial. But here we have them. And we have meta-analyses.
Lamberti:
Most of the clinical trials are small and poorly done.
MacIntyre:
They actually did do an RCT in parachute use that was just published.7
†Hess:
Right, it depends on whether the airplane is on the ground or in the air.
MacIntyre:
What was cool about it is that plane location wasn’t listed as one of the major results. It was only a ‘limitation’ of the study. Nobody would volunteer to be in the control group unless the plane was on the ground.
†Hess:
In the ASA guideline,8 did they make any suggestions as to how capnography should be used? Are they suggesting that it be used as a breathing frequency monitor, and I get that, or do they recommend that it be used to monitor end-tidal PCO2, or both of those things?
Lamberti:
The ASA guideline states that capnography should be used to continuously “monitor ventilatory function.” I believe that the guideline is focusing on breathing frequency monitoring. There is no specific mention of end-tidal PCO2 measurement.
†Hess:
If they are suggesting it as a monitor for breathing frequency, then it’s consistent with what Brady [Scott] talked about yesterday.
Lamberti:
Yes, and there are certainly other ways to measure breathing frequency. The ASA guideline focused on capnography and did not speak to the other modalities of ventilation monitoring such as acoustic sensor, transcutaneous PCO2, airflow detector and impedance plethysmography.
Dexter:
I want to go back to Craig’s [Rackley] comment about how clinicians are expected to properly manage and monitor these difficult patients given the labor-intensive patient assignments we receive at shift change. We’re starting to use telemedicine a lot more in the Charlotte area and RTs are starting to be incorporated into the telemedicine teams. I wonder if we should be using telemedicine teams not only in the ICU setting, but transitioning that concept to the general care floors as well?
Lamberti:
I think that’s a good point. There’s now mobile technology different than the eICU from 15 years ago where it was a fixed camera and room. There are now mobile telemonitoring solutions that can go into a patient’s room.
MacIntyre:
Let me follow up on Amanda’s [Dexter] point on telemedicine. I think the evidence is good, though I don’t know it well. Monitoring electrocardiograms remotely in high-risk patients really pays off in the general care unit. Why not expand that to respiratory parameters?
Lamberti:
There are a lot of data about unnecessary electrocardiographic (ECG) telemonitoring. What has happened with ECG telemetry is that anybody who’s sick gets put on telemetry and ECG monitoring, so it really doesn’t help that much. If you have severe pneumonia, you have a heart rate of 110 beats/min. If your heart rate increases to sinus tachycardia at a rate of 120 beats/min, that doesn’t get anybody’s attention. I’m not yet sure what to say is the best monitoring technique for respiratory patients. End tidal PCO2 has a lot of problems with regards to how it’s measured; is it the breathing frequency, end tidal PCO2, or shape of the capnogram that we should utilize? One manufacturer has developed an integrated pulmonary index to simplify monitoring.9 It’s a tough question.
Scott:
It is a tough question. When putting an EKG on someone, it’s just electrode stickers. For the most part, patients can go about their day with slight difficulty with the wires. The problem with end tidal PCO2 is that it’s in their nose. When they move around, it increases the rate of alarms, there are false alarms, and sometimes it is totally removed by the patient. Sometimes patients just can’t tolerate it. That’s a big issue and perhaps eventually we will have technological advances that makes these devices more comfortable and wearable. I don’t know the data on EKGs, but I think what makes it easy, is that it doesn’t really interfere with anything versus the cannula of the capnograph.
Lamberti:
We tell our patients to get out of bed and move around but then we hook them up to monitors that require physical connections.
†Hess:
There are things about looking at the end tidal PCO2 during procedural sedation that are really tricky and not appreciated. If you lower your minute ventilation by lowering the breathing frequency but maintaining your tidal volume, the end tidal PCO2 goes up. But if you lower your minute ventilation by lowering your tidal volume, your dead space goes up and your end tidal PCO2 goes down. You mentioned that on one of your slides where you indicated that the device alarmed or alerted for either an increase or a decrease in end-tidal PCO2. There’s a physiologic disconnect there in a lot of people’s minds, where they can’t get their arms around the fact that the end-tidal PCO2 could ge going down and the patient is getting into trouble.
Lamberti:
Absolutely correct Dean. The recently published trial of monitoring in opioid-induced respiratory depression utilized a definition of respiratory depression of “end-tidal PCO2 ≤15 or ≥ 60 mm Hg for ≥ 3 min.”
†Hess:
An emergency department physician and I published a paper10 more than 10 years ago where we described this, and the peer reviewers were pretty rough on us. Eventually it got published and there have now been a number of papers from the procedural sedation literature, mostly from the emergency department, showing that many times patients who get into trouble when their end-tidal PCO2 goes down because they breath rapid and shallow.
Scott:
I think that’s an important point. I spent several years working primarily in an emergency department and was involved in procedural sedation. Sometimes I watched the frequency and waveform, more than the actual end tidal PCO2. If the end tidal PCO2 went up or down, I wasn’t sure what to do with that information at times. But it is complicated, it’s not as easy as you think, it doesn’t just go up when someone is in trouble. Sometimes it goes down and they’re actually in a lot of trouble.
Walsh:
One more thing is that end tidal PCO2 may be more accurate at counting frequency, especially in obstruction. A lot of times you can have EKG movement of the breathing frequency but you won’t have an end tidal PCO2 change in frequency so that’s something else the anesthesia literature supports is that it may be more accurate than the EKG frequency.
Footnotes
- Correspondence: James P Lamberti MD, Department of Medicine, Inova Fairfax Hospital, Falls Church, Virginia. E-mail: james.lamberti{at}inova.org
Dr Lamberti has disclosed relationships with Boehringer Ingelheim, GlaxoSmithKline, Janssen, Philips, Portola, and Sunovion.
Dr Lamberti presented a version of this paper at the 58th Respiratory Care Journal Conference, Monitoring Respiratory Function in Adult Acute Care, held June 10–11, 2019, in St Petersburg, Florida.
↵* Timothy R Myers MBA RRT RRT-NPS FAARC is Chief Business Officer of the AARC.
↵† Dean R Hess PhD RRT FAARC is Managing Editor of Respiratory Care.
- Copyright © 2020 by Daedalus Enterprises