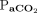Abstract
BACKGROUND: Apnea testing is the last step of brain death assessment. This study aimed to determine whether apnea testing is safer when performed over a shorter duration.
METHODS: The medical records of 200 brain-dead donors were retrospectively evaluated. All the records were anonymously registered in the Japanese Ministry of Health, Labor, and Welfare from 1999 to 2012. The rate of  increase was analyzed to calculate the duration required for apnea testing.
increase was analyzed to calculate the duration required for apnea testing.
RESULTS: At baseline, body temperature and  significantly affected the increase rate of
significantly affected the increase rate of 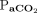 . At baseline, the apnea testing durations were 4.7 min with normal body temperature and higher
. At baseline, the apnea testing durations were 4.7 min with normal body temperature and higher 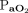 (
(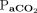 40–60 mm Hg, body temperature 36.5°C,
40–60 mm Hg, body temperature 36.5°C, 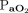 400 mm Hg); further, it was 3.0 min with higher body temperature and lower
400 mm Hg); further, it was 3.0 min with higher body temperature and lower  at baseline (
at baseline (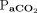 40–60 mm Hg, body temperature 38.0°C,
40–60 mm Hg, body temperature 38.0°C, 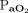 100 mm Hg).
100 mm Hg).
CONCLUSIONS: The specific duration of apnea testing during brain death assessment may be predicted by measuring the increase rate of 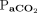 .
.
Introduction
Apnea testing is a crucial determinant in brain death diagnosis; however, it can be an invasive procedure. Although most apnea testing procedures can be safely performed with intensive monitoring, it can lead to complications, including hypotension, hypoxemia, and acidemia.1–3 The Japanese Society of Anesthesiology Guidelines for the Implementation of Apnea Test (https://anesth.or.jp/files/pdf/guideline_MukokyuTest.pdf, Accessed March 1, 2020) specify that apnea testing should be continued until the 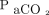 reaches 60 mm Hg. However, apnea testing duration seems unpredictable.4–7 Calculating the increase in
reaches 60 mm Hg. However, apnea testing duration seems unpredictable.4–7 Calculating the increase in 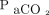 during apnea testing could allow for greater control and a shorter testing period, thus improving patient safety. However, this topic has not been studied. Therefore, we aimed to test the hypothesis that apnea testing would be safer if performed over a shorter duration and may prevent complications such as hypoxemia and hypotension.
during apnea testing could allow for greater control and a shorter testing period, thus improving patient safety. However, this topic has not been studied. Therefore, we aimed to test the hypothesis that apnea testing would be safer if performed over a shorter duration and may prevent complications such as hypoxemia and hypotension.
Quick look
Current Knowledge
Apnea testing is often safely performed with intensive monitoring; however, complications such as hypotension, hypoxemia, and acidemia can occur. So far, apnea testing duration has been considered unpredictable.
What This Paper Contributes to Our Knowledge
Apnea testing duration may be predicted using a calculation based on the baseline body temperature and 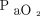 level, which are correlated with the increase rate of
level, which are correlated with the increase rate of  . Predicting the duration of apnea testing may reduce unnecessarily prolonged apnea testing procedures that result in severe complications that compromise organ donation.
. Predicting the duration of apnea testing may reduce unnecessarily prolonged apnea testing procedures that result in severe complications that compromise organ donation.
Methods
We reviewed 200 consecutive cases of individuals who experienced brain death and had donated their organs between 1999 and 2012 (Table 1). Data were obtained from the Japanese Ministry of Health, Labor, and Welfare (MHLW), Summary of Verification Meeting on Cases of Organ Donation under Brain Death (https://www.mhlw.go.jp/seisakunitsuite/bunya/kenkou_iryou/kenkou/zouki_ishoku/dl/200_matome.pdf, Accessed March 1, 2020) and included records from medical institutions in Japan. These institutions reported sufficient systems for organ donation, as confirmed by the MHLW. The results of blood gas analysis during apnea testing were recorded to allow calculation of the increase rate of the  . For all subjects with brain death, we reviewed data regarding blood gas levels at baseline and at subsequent 2- to 3-min intervals until
. For all subjects with brain death, we reviewed data regarding blood gas levels at baseline and at subsequent 2- to 3-min intervals until 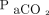 levels reached 60 mm Hg.
levels reached 60 mm Hg.
Causes of Brain Death in Japanese Subjects: 1999–2012
Modeling and Simulations to Analyze the Increase in 
We attempted to model how 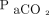 increases using nonlinear mixed-effects modeling software (NONMEM 7.3, ICON, Dublin, Ireland). We assumed that the increase ratio of
increases using nonlinear mixed-effects modeling software (NONMEM 7.3, ICON, Dublin, Ireland). We assumed that the increase ratio of 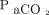 in the structure models was as follows:
in the structure models was as follows:
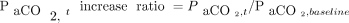
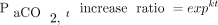 (A)
(A)
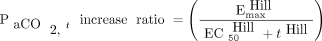 (B) where equation A assumes a first-order increasing model, whereas equation B assumes a sigmoid Emax model.
(B) where equation A assumes a first-order increasing model, whereas equation B assumes a sigmoid Emax model. 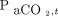 is the
is the 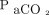 at time t after apnea testing initiation (mm Hg), k is the
at time t after apnea testing initiation (mm Hg), k is the 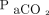 increasing rate constant (/min), t is the time after apnea testing initiation (min), Emax is the maximum
increasing rate constant (/min), t is the time after apnea testing initiation (min), Emax is the maximum  (mm Hg), EC50 is the time after apnea testing initiation required to reach half the
(mm Hg), EC50 is the time after apnea testing initiation required to reach half the  ratio up to Emax (min), and Hill is the sigmoid curve shape. We chose the simplest and most visually appropriate model of the 2 models, with the objective function value (a lower value is better for fitting the observation) being calculated during the NONMEM analysis. The exponential distribution assigned the inter-individual variability.
ratio up to Emax (min), and Hill is the sigmoid curve shape. We chose the simplest and most visually appropriate model of the 2 models, with the objective function value (a lower value is better for fitting the observation) being calculated during the NONMEM analysis. The exponential distribution assigned the inter-individual variability.
Next, we explored covariates that affect the increase ratio of 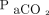 . Available individual data included age, sex,
. Available individual data included age, sex,  , body temperature, systolic blood pressure, diastolic blood pressure, pupil diameters, and auditory brainstem response test results. The Pearson test was used to determine the significance of the correlation of continuous variables with the
, body temperature, systolic blood pressure, diastolic blood pressure, pupil diameters, and auditory brainstem response test results. The Pearson test was used to determine the significance of the correlation of continuous variables with the 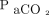 increasing rate constant. Here, variables with a correlation coefficient > 0.2 and P < .05 were considered as potential covariates. The significance of the categorical variables was examined using the Fisher exact test, with P < .05 indicating potential covariates. Next, we developed single-covariate models using potential covariates during the forward inclusion steps. Continuous and categorical variables were modeled for allometry and power, respectively. Based on the theory that objective function values follow a chi-square distribution, a reduction in the objective function value by > 3.84 can serve as a reference for significance (P < .05) when a potential covariate is included. Subsequently, a full model was developed using potential significant covariates during the forward inclusion steps. The best model was finalized by testing the full model through backward elimination, where each covariate that increased the objective function value by 6.63 (P > .01) upon removal was eliminated.
increasing rate constant. Here, variables with a correlation coefficient > 0.2 and P < .05 were considered as potential covariates. The significance of the categorical variables was examined using the Fisher exact test, with P < .05 indicating potential covariates. Next, we developed single-covariate models using potential covariates during the forward inclusion steps. Continuous and categorical variables were modeled for allometry and power, respectively. Based on the theory that objective function values follow a chi-square distribution, a reduction in the objective function value by > 3.84 can serve as a reference for significance (P < .05) when a potential covariate is included. Subsequently, a full model was developed using potential significant covariates during the forward inclusion steps. The best model was finalized by testing the full model through backward elimination, where each covariate that increased the objective function value by 6.63 (P > .01) upon removal was eliminated.
The 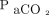 increase ratio was simulated for each representative value identified as a covariate in the final model using R 3.6.1 (R, Vienna, Austria).
increase ratio was simulated for each representative value identified as a covariate in the final model using R 3.6.1 (R, Vienna, Austria).
Standard Protocol and Subject Consent
This study was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The institutional review board of the Kumamoto University Hospital waived the need for informed consent given the retrospective nature of this study. Data obtained from the Japanese MHLW lacked identifying patient information. Furthermore, subject data will not be shared because the Japanese MHLW provided exclusive permission for this study to use the data for analyses.
Results
Development of the Final Model
Structure model A (exponential model) was used because the objective function value was 1963.496, whereas that of model B (sigmoid Emax model) was 3546.420. Subsequently, the potential covariates were tested, with baseline 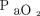 and temperature at initiation meeting the criteria for developing a full model. Although the significance of other covariates was investigated during the backward elimination step, these 2 covariates remained the most significant. Next, a final model was determined for the increase in
and temperature at initiation meeting the criteria for developing a full model. Although the significance of other covariates was investigated during the backward elimination step, these 2 covariates remained the most significant. Next, a final model was determined for the increase in  (Fig. 1). Baseline
(Fig. 1). Baseline 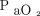 and body temperature were significant factors, with a lower baseline
and body temperature were significant factors, with a lower baseline 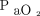 and higher baseline body temperature (Fig. 1) being significantly correlated with a greater increase in the
and higher baseline body temperature (Fig. 1) being significantly correlated with a greater increase in the  rate during apnea testing. The following regression equation for predicting the necessary apnea testing duration was developed:
rate during apnea testing. The following regression equation for predicting the necessary apnea testing duration was developed:
 where duration (in min) = ln(
where duration (in min) = ln(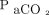 increase ratio)/[0.0863 × (body temp/36.5)2.09 × (
increase ratio)/[0.0863 × (body temp/36.5)2.09 × (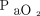 /400)−0.16] and
/400)−0.16] and 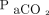 increase ratio =
increase ratio =  . Here,
. Here,  refers to the
refers to the 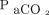 level at the end of apnea testing, and
level at the end of apnea testing, and 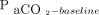 refers to the baseline
refers to the baseline 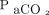 level; body temperature is in degrees Celsius.
level; body temperature is in degrees Celsius.
Models for predicting  increase. (A) Measured proportion of the
increase. (A) Measured proportion of the  increase across time intervals. (B) Correlation between the measured proportion and predicted proportion of the
increase across time intervals. (B) Correlation between the measured proportion and predicted proportion of the  increase. (C) Correlation between the measured
increase. (C) Correlation between the measured 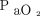 and
and 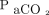 increasing constant (k). (D) Correlation between body temperature and the increasing constant (k).
increasing constant (k). (D) Correlation between body temperature and the increasing constant (k).
All  levels were measured in mm Hg. Figure 2 describes the mean values and their corresponding 64% prediction intervals. The time required for
levels were measured in mm Hg. Figure 2 describes the mean values and their corresponding 64% prediction intervals. The time required for 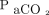 to increase from 40 mm Hg to 60 mm Hg (
to increase from 40 mm Hg to 60 mm Hg (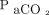 increase ratio = 1.5) at 4 representative values (with 64% prediction intervals) of temperature and
increase ratio = 1.5) at 4 representative values (with 64% prediction intervals) of temperature and  at apnea testing initiation were 4.7 min (3.4–6.4; Fig. 2A), 3.8 min (2.8–5.2; Fig. 2B), 4.3 min (3.2–6.0; Fig. 2C), and 3.0 min (64% PI: 2.5–4.8; Fig. 2D).
at apnea testing initiation were 4.7 min (3.4–6.4; Fig. 2A), 3.8 min (2.8–5.2; Fig. 2B), 4.3 min (3.2–6.0; Fig. 2C), and 3.0 min (64% PI: 2.5–4.8; Fig. 2D).
Predicted proportion of  increase at representative temperatures and
increase at representative temperatures and 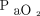 values at the start of apnea testing. The black curve is the predicted curve for a body temperature of 36.5°C and
values at the start of apnea testing. The black curve is the predicted curve for a body temperature of 36.5°C and 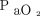 of 400 mm Hg at baseline (A). Blue curves and shaded areas show predicted curves and corresponding 64% prediction intervals, respectively, of the representative values indicated in each figure (B, for a body temperature of 36.5°C and
of 400 mm Hg at baseline (A). Blue curves and shaded areas show predicted curves and corresponding 64% prediction intervals, respectively, of the representative values indicated in each figure (B, for a body temperature of 36.5°C and 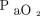 of 100 mm Hg at baseline; C, for a body temperature of 38.0°C and
of 100 mm Hg at baseline; C, for a body temperature of 38.0°C and 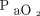 of 400 mm Hg at baseline; D, for a body temperature of 38.0°C and
of 400 mm Hg at baseline; D, for a body temperature of 38.0°C and 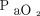 of 100 mm Hg at baseline).
of 100 mm Hg at baseline).
Discussion
Apnea testing, which is necessary for brain death assessment, can be invasive. Therefore, quick termination of apnea testing after fulfilling the test criteria would be preferred. Herein, a formula was developed to calculate the minimum apnea testing duration. During most brain death assessments, apnea testing is completed within 8–10 min.5,6 However, the Summary of Verification Meeting on Cases of Organ Donation under Brain Death from the Japanese MHLW (https://www.mhlw.go.jp/seisakunitsuite/bunya/kenkou_iryou/kenkou/zouki_ishoku/dl/200_matome.pdf, Accessed March 1, 2020) indicates that apnea testing is typically completed within 6 min (mean duration = 5.7 min). However, the American Academy of Neurology8 and Japanese guidelines9 differ with respect to when to start and repeat blood gas analyses during apnea testing. Specifically, the American and Japanese guidelines stipulate starting after 8 min and after 2 or 3 min, respectively. Furthermore, the American Academy of Neurology guidelines do not indicate a repeat interval, whereas the Japanese guidelines indicate a repeat interval of 2 or 3 min after initiation.
Similarly, there appears to be a discrepancy in the mean 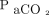 increase. Global measurements10 range between 2.5 and 3.0 mm Hg/min; however, the Results and Problems in Brain Death Assessment in Japan (https://www.jstage.jst.go.jp/article/jst/48/2-3/48_89/_pdf, Accessed March 1, 2020) indicated that the average was 4.7 mm Hg/min. This discrepancy could be attributed to the inverse relationship between the mean
increase. Global measurements10 range between 2.5 and 3.0 mm Hg/min; however, the Results and Problems in Brain Death Assessment in Japan (https://www.jstage.jst.go.jp/article/jst/48/2-3/48_89/_pdf, Accessed March 1, 2020) indicated that the average was 4.7 mm Hg/min. This discrepancy could be attributed to the inverse relationship between the mean 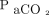 increase and apnea testing duration. The precise
increase and apnea testing duration. The precise 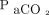 increase appears to be nonlinear5 and unpredictable.7 Another report identified possible variations in the
increase appears to be nonlinear5 and unpredictable.7 Another report identified possible variations in the 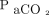 increase and stated that it was associated with factors including baseline
increase and stated that it was associated with factors including baseline 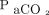 , oxygen flow delivery, and body temperature.5 Among these factors, oxygen flow delivery is usually fixed at 6 L/min. In the formula we developed, the increase ratio of the
, oxygen flow delivery, and body temperature.5 Among these factors, oxygen flow delivery is usually fixed at 6 L/min. In the formula we developed, the increase ratio of the 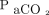 was defined as the
was defined as the  value at the end of the testing period divided by the baseline
value at the end of the testing period divided by the baseline  . Because body temperature and baseline
. Because body temperature and baseline 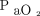 may significantly affect the increase rate of the
may significantly affect the increase rate of the  , the testing duration may be shortened by both higher body temperature and lower
, the testing duration may be shortened by both higher body temperature and lower  at baseline.
at baseline.
Higher body temperatures could be assumed to boost the  increase due to increased CO2 production during metabolism. However, the mechanism underlying the effect of baseline
increase due to increased CO2 production during metabolism. However, the mechanism underlying the effect of baseline 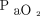 on apnea testing duration remains unclear. In this study, individuals who demonstrated no increase in baseline
on apnea testing duration remains unclear. In this study, individuals who demonstrated no increase in baseline  after preoxygenation with 100% oxygen could have developed moderate or severe lung injury.11 Severely injured lungs may have reduced gas exchange and impaired CO2 removal. Using an oxygen insufflation method, Kramer et al12 reported that oxygen flow through a catheter to the endotracheal tube eliminated CO2. Therefore, for individuals with impaired CO2 removal, the
after preoxygenation with 100% oxygen could have developed moderate or severe lung injury.11 Severely injured lungs may have reduced gas exchange and impaired CO2 removal. Using an oxygen insufflation method, Kramer et al12 reported that oxygen flow through a catheter to the endotracheal tube eliminated CO2. Therefore, for individuals with impaired CO2 removal, the  increase could be higher and apnea testing can be terminated earlier.
increase could be higher and apnea testing can be terminated earlier.
The retrospective design of this study, as well as the use of restricted data that are not publicly available, limited our ability to determine the precise lung diseases possibly associated with baseline  . This can be explored in future studies. Moreover, we have not confirmed the validity and reliability of our formula for predicting the apnea testing duration during brain death assessment. However, we hope to do so in future studies.
. This can be explored in future studies. Moreover, we have not confirmed the validity and reliability of our formula for predicting the apnea testing duration during brain death assessment. However, we hope to do so in future studies.
We believe that predicting the necessary minimum duration of apnea testing could reduce the strain on practitioners who perform apnea testing and help preserve vital organs for donation. Specifically, predicting the apnea testing duration may reduce the chance of unnecessarily prolonged apnea testing procedures, which can cause potential complications, including hypotension, hypoxemia, and acidemia.
Conclusions
Specific apnea testing durations during assessment of brain death may be predicted by measuring the rate of increase in  . Furthermore, the minimum apnea testing duration may be predicted using a calculation based on the baseline body temperature and
. Furthermore, the minimum apnea testing duration may be predicted using a calculation based on the baseline body temperature and 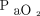 level, which are correlated with the rate of increase in
level, which are correlated with the rate of increase in 
Acknowledgments
The authors thank the Japanese Ministry of Health, Labour, and Welfare for providing the data used for this study. The authors also thank Kazutaka Oda PhD, of Kumamoto University Hospital, Department of Pharmacy, for conducting the statistical analysis. Finally, the authors thank Editage (www.editage.com) for English language editing.
Footnotes
- Correspondence: Katsuyuki Sagishima MD. E-mail: saggy{at}kuh.kumamoto-u.ac.jp
The authors have disclosed no conflicts of interest.
- Copyright © 2021 by Daedalus Enterprises